Abstract
Bats, rodents and monkeys are reservoirs for emerging zoonotic infections. We sought to describe the frequency of human exposure to these animals and the seasonal and geographic variation of these exposures in Bangladesh. During 2013–2016, we conducted a cross-sectional survey in a nationally representative sample of 10,002 households from 1001 randomly selected communities. We interviewed household members about exposures to bats, rodents and monkeys, including a key human–bat interface–raw date palm sap consumption. Respondents reported observing rodents (90%), bats (52%) and monkeys (2%) in or around their households, although fewer reported direct contact. The presence of monkeys around the household was reported more often in Sylhet division (7%) compared to other divisions. Households in Khulna (17%) and Rajshahi (13%) were more likely to report drinking date palm sap than in other divisions (1.5–5.6%). Date palm sap was mostly consumed during winter with higher frequencies in January (16%) and February (12%) than in other months (0–5.6%). There was a decreasing trend in drinking sap over the three years. Overall, we observed substantial geographic and seasonal patterns in human exposure to animals that could be sources of zoonotic disease. These findings could facilitate targeting emerging zoonoses surveillance, research and prevention efforts to areas and seasons with the highest levels of exposure.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10393-023-01628-9.
Keywords: Human exposure, Bat, Rodent, Monkey, Bangladesh
Introduction
Most emerging infectious diseases in humans are caused by zoonotic pathogens, with the majority (72%) originating from wildlife (Jones et al. 2008). Pathogens originating from bats, rodents and non-human primates have caused outbreaks and pandemics in humans, including human immunodeficiency virus infection from primates (Wolfe et al. 2007), Ebola from primates and bats (Wolfe et al. 2007; Leroy et al. 2009; Qiu et al. 2014), Nipah, severe acute respiratory syndrome (SARS) (Calisher et al. 2006), SARS coronavirus 2 (SARS-CoV-2) (Holmes et al. 2021) from bats, hantavirus pulmonary syndrome (CDC, 2017), Lassa fever (CDC, 2017) and bubonic plague from rodents (Arya 1994). Pathogen spillover into humans depends on many factors, but human exposure is a necessary step in the process (Plowright et al. 2017). Therefore, identifying areas where contact between humans and these animals is most frequent may be an efficient way to target surveillance and investigation for pathogen spillover.
Bangladesh is a hotspot for zoonotic disease emergence because of its extremely dense human population (1252 people per km2) (World Bank, 2017), diverse wildlife population (Khan, 2008), increasing urbanization and deforestation (South Asia Environment Outlook 2009), and vulnerability to climate change (Anthony et al. 2015; Kabir et al. 2016). Zoonotic pathogens of bat, rodent and primate origin have all been reported in Bangladesh (Kendall et al. 2010; Rahman et al. 2012; Anthony et al. 2015). Nipah virus is the most fatal of these infections; in Bangladesh, 322 human cases of Nipah infection were reported between 2001 and 2021 and 71% (229/322) died (IEDCR, 2021). Nipah virus is a stage III pathogen meaning that it can spill over from animals to humans and also cause person-to-person transmission (Luby SP 2013). An estimated 33% of human Nipah virus infections in Bangladesh resulted from person-to-person transmission, even though only 9% of all cases infected someone else (Nikolay et al. 2019). If Nipah virus becomes more transmissible, it could pose a risk for larger outbreaks or a pandemic. Bat-to-human transmission of Nipah virus in Bangladesh has been driven by the consumption of raw date palm sap contaminated with the saliva or urine of Pteropus medius bats (previously P. giganteus) (Luby et al. 2006; Salah Uddin Khan et al. 2010; Rahman et al. 2012; Hegde et al. 2016; Epstein et al. 2020). Both fruit bats P. medius, the reservoir host of Nipah virus, and date palm trees (Phoenix sylvestris) are widely distributed throughout the country (Rahman et al. 2012; Epstein et al. 2020). In rural Bangladesh, the consumption of fresh raw date palm sap early in the morning during winter (usually November–February) is a common practice (Gurley et al. 2007; Nahar et al. 2010; Sazzad et al. 2013). Date palm sap harvesters (local name gachhis) usually collect raw sap during the cold season by shaving the bark of the tree and hanging a pot to collect the sap overnight (Nahar et al. 2010). Sometimes, they use skirt-like barriers, locally called bana, to cover the shaved area and the collection pot to prevent access by bats, rodents, birds and insects (Nahar et al. 2010).
Zoonotic pathogens from rodents, including Leptospira, hantavirus, Yersinia pestis also infect humans in Bangladesh and surrounding India. Humans can acquire infections during direct contact with infected rodents or can be infected by food or household items contaminated with rodent droppings or urine (Meerburg et al. 2009). A study in a low-income urban community in Dhaka, Bangladesh, in 2001 reported that 11% (n = 584) of febrile patients’ convalescent-phase serum samples had IgM antibodies against Leptospira; 2.2% were confirmed to have leptospirosis by the microscopic agglutination test (MAT) (Kendall et al. 2010). Other rodent-borne infections were detected in neighboring India, which shares many ecologic and cultural similarities to Bangladesh. Among 99 rat consumers and professional rat catchers from a tribal community in Tamil Nadu, a state in south India, 11% had IgM antibodies against hantavirus, suggesting recent infection (Chandy et al. 2008). In the Himachal state of northern India, a localized outbreak of pneumonic plague occurred in 2002 (Butler 2009) and an outbreak of bubonic plague was reported in Uttarakhand in 2004 (Biswas 2018). In Bangladesh, we know of no efforts to identify human infections with either hantavirus nor plague so it is possible that these have gone unnoticed by public health.
Non-human primates, including Rhesus macaques (Macaca mulatta), are common in many rural and urban areas of Bangladesh and are sometimes kept by humans for street or circus performance purposes (Hasan et al. 2013; Feeroz et al. 2013). Primates may move into areas of human settlements due to decreasing habitat from deforestation and come into physical contact with humans while seeking out food (Shano et al. 2021). A number of potentially zoonotic pathogens have been detected in rhesus macaques in Bangladesh, including rotavirus, adenovirus and herpesvirus (Anthony et al. 2015; Islam et al. 2020), and direct contact with rhesus macaques has led to the transmission of simian foamy viruses (SFVs) to humans (Feeroz et al. 2013).
A better understanding of animal exposure in Bangladesh may assist in targeting surveillance and research aimed at detecting and preventing emerging zoonotic infections in humans. Our study aimed to quantify the frequency and describe the geographical and seasonal variation in human exposure to bats, rodents and monkeys, including consumption of date palm sap.
Methods
Study Design
We conducted a nationally representative cross-sectional survey in Bangladesh from September 2013 to August 2016. We balanced the enrollment of communities to enable comparisons between geographical regions and seasons. In each of the three seasons (winter: November–February, summer: March–June and rainy: July–October) (Haider et al. 2014; Moniruzzaman 2015), we enrolled 16 new communities (defined as villages in rural areas and wards in urban areas) from each of the seven administrative divisions (first-level administrative unit) in Bangladesh. To select communities for the survey, we first used data from the 2011 census to divide communities by division, then categorized communities into 16 strata based on the size of the community, and with an equal number of communities in each stratum, such that each stratum contained communities of relatively homogeneous sizes. We took this step of categorizing communities by size because we hypothesized that exposures to wildlife might be different in smaller, more remote communities and we wanted to ensure those were included in our sample.
Sample Size Calculation
We aimed to have 10% absolute precision and 95% confidence in the fraction of people exposed to wildlife. Since we had limited information about humans' exposure to wildlife in Bangladesh, we conservatively assumed that 50% of households would have at least one person exposed to one of these three groups of wildlife within the past month and this assumption would generate the maximum required sample size. To meet these analytical goals with these assumptions, our required sample size was of 97 households (assuming a binomial distribution) for each division during each season. After considering a 5% non-response rate and design effect of 1.5 to account for the non-independence of sampling units (households) within a community (Kaiser et al. 2006; Alimohamadi and Sepandi 2019), our sample size was 153 households per division per season, which we rounded up to 160 to target 10 households from each of the 16 population strata. In total, our estimated sample size was approximately 3360 households across Bangladesh (7 divisions × 16 communities × 10 households × 3 seasons) each year, giving a total of 10,080 households over 3 years.
Household Selection from Rural Areas
GPS coordinates were taken for each sampled community. In each rural community (village), we enrolled 10 randomly selected households. To identify a random starting point for the survey in each village, our study team first identified the homestead (typically consists of 4–5 households where members are usually relatives) where the most recent wedding took place; this served as a random starting location in the village as nearly all adults marry and marriage patterns are very similar throughout the country (BBS, 2015). The first household to the left of the main entrance of that homestead was then selected for enrollment in the study. After enrollment of the first household, we skipped the next two closest homesteads to the right and entered the third closest homestead to select the 2nd household. This process was repeated until 10 households from 10 homesteads were enrolled from the selected community. If any homestead declined to participate, the team selected the next closest homestead. If the team arrived at the edge of the community before enrolling 10 households, they returned to the starting point and moved toward the left following the same selection criteria.
Household Selection from Urban Areas
We used a different a process to select households from urban communities (wards) due to the increased density of dwellings, including apartment buildings and single households compared to rural areas. The team carried four separate envelopes containing papers on which each of the four cardinal directions was written: east, west, south or north. To select the first dwelling, the team randomly picked one envelope and followed the direction written inside to the administrative boundary of that ward and selected the nearest dwelling to that point. If a multilevel apartment building was the closest dwelling to that point, the team used a random number table to select the floor from which to select a household for enrollment. If there were multiple apartments on the selected floor, the team selected the apartment immediately on the left of the stairwell to enroll in the study. After enrolling the first household, the team skipped the next four closest dwellings (apartment buildings or single households) to select the next household for enrollment. The team followed the same procedures as in rural areas if any household declined to participate.
Data Collection
From each selected household in both rural and urban communities, the team identified the most senior household member aged between18–60 years who was available and willing to provide information about themselves and other household members. A member of the study team used a structured questionnaire to ask enrolled participants about their household characteristics and household members’ exposures to rodents, bats and monkeys and domestic animals within the past month and also inspected the areas visible from household premises to identify bat roosts and fruit trees growing on the homestead. The team also asked household members about the consumption of date palm sap and fruit that appeared to have been bitten by an animal since these could also expose humans to fruit bat pathogens.
Statistical Analysis
We used descriptive statistics to summarize household and community exposures to wildlife and estimated the prevalence of each exposure with 95% CIs, adjusting for the clustering of households within each sampled community. We compared exposures by divisions and seasons by comparing the point estimates with CIs and Z-test with p value at 5% level of significance. We plotted the proportion of households reporting raw date palm sap consumption by year (November to April) throughout the study period and used a chi-squared trend test to identify significant changes. Participants were asked about their exposures within the last month before the survey, so operationally, surveys that were conducted from the 1st through the 10th day of the month were categorized as exposures for the previous calendar month in the analyses. We mapped the location (latitude, longitude) of each study community with exposure history to examine spatial patterns in exposure to wildlife. We used Stata SE (StataCorp. 2013. Stata Statistical Software: Release 13. College Station, TX: StataCorp LP.) for data analysis and R Statistical Software (V4.2.2; R Core Team 2022) via the ‘maptools’ R package (V1.1.6; Bivand R, Lewin-Koh N 2022), the ‘sp’ R package (V1.6.0; Bivand RS, Pebesma E, Gomez-Rubio V 2013) and the ‘ggplot2’ R package (V3.4.1; Wickham H 2016) for creating maps and graphs.
Operational Definitions
We have defined different types of human exposures to animals. Survey respondents were asked about the consumption of date palm sap by any household member within the past month, as this is a common exposure pathway for transmission of Nipah virus infection from bats to humans (Luby et al. 2006; Epstein et al. 2020). Direct contact with bats, rodents or monkeys was defined as a report that any household member had physical contact with living or dead bats (of any size), rodents or monkeys, including touching, playing, capturing, hunting, consuming or being bitten, attacked or scratched by these animals within the past month. The presence of bats, rodents or monkeys in or around the household was defined as a report that any household member saw bats or monkeys nearby the household or saw rodents in their household within the past month. We determined that bats, rodents or monkeys were present in the community if at least one household respondent in the community reported seeing bats or monkeys nearby the household, or seeing rodents in their household within the past month. Among the 10 species of primates in Bangladesh, the most common is the rhesus macaque, commonly known as “banor” or “bandor” and this term was used in our study; however, we assume that respondents may have used this Bengali word to refer to any primate species.
Human Subjects Considerations
We obtained written, informed consent from each participant before administering the questionnaire. The protocol was reviewed and approved by icddr,b’s research review and ethical review committees (protocol number # PR-13033).
Results
We approached 10,277 households and enrolled participants from 10,002 households (2% declined to participate) from 1001 communities. We initially targeted enrollment in 1008 communities, but were unable to visit the last 7 due to financial constraints. The median number of family members per household was 5 (interquartile range [IQR] 4–6) in rural areas and 4 in urban areas (IQR 4–6). The percentages of households that reported having domestic animals or fruit trees, consuming dropped fruit and fruit previously bitten by an animal and consuming date palm sap were significantly higher in rural compared to urban areas (Table 1).
Table 1.
Characteristics of selected households and percentages of households in which at least one household member had exposures to wild animals (rodents, bats or monkeys) in the past month in Bangladesh, 2013 to 2016 (N = 10,002).
| Household characteristics, practices and exposure to wild animals | Overall percentage (95% CI) | Rural N = 8294 percentage (95% CI) |
Urban N = 1708 percentage (95% CI) |
Differences (rural vs. urban) percentage (p-value) |
|---|---|---|---|---|
| Having domestic animals in the household | 54 (53–55) | 61 (60–62) | 21 (19–23) | 40 (< 0.001) |
| Having any fruit tree near the boundary of the household | 95 (94–95) | 98 (97.8–98.4) | 78 (76–80) | 20 (< 0.001) |
| Any of the household members having consumed dropped fruit | 33 (32–34) | 37 (35–38) | 17 (15–19) | 20 (< 0.001) |
| Any of the household members having eaten fruit previously bitten by an animal | 13 (12–14) | 15 (14–15) | 5 (4–6) | 10 (< 0.001) |
| Any of the household members having drank fresh/raw date palm sap (throughout the study period) | 3.71(3.4–4.1) | 3.96 (3.5–4.4) | 2.5 (1.8–3.2) | 1.46 (0.055) |
| Used a method of rodent control in your house or property | 20 (19–21) | 21 (20–21) | 18 (16–19) | 3 (0.013) |
| Any household member had physical contact with rodents and/or observed rodents in the household | 90 (89–91) | 92 (91–92) | 84 (82–86) | 8 (< 0.001) |
| Rodents were observed in the household | 90 (90–91) | 92 (91–92) | 84 (82–86) | 8 (< 0.001) |
| Rodents were touched by household members | 8.53 (8.0–9.1) | 8.61 (8.0–9.2) | 8.14 (6.8–9.4) | 0.005 (0.537) |
| Any household member had physical contact with bats, including hunting or consuming | 0.5 (0.4–7) | 0.6(0.4–0.7) | 0.35 (0.1–0.7) | 0.25 (0.285) |
| Any household member touched, bitten or scratched by a monkey | 0.05 (0.02–0.13) | 0.05 (0.01–0.1) | 0.06 (0.056–0.17) | − 0.01 (0.876) |
Presence of and Exposure to Bats
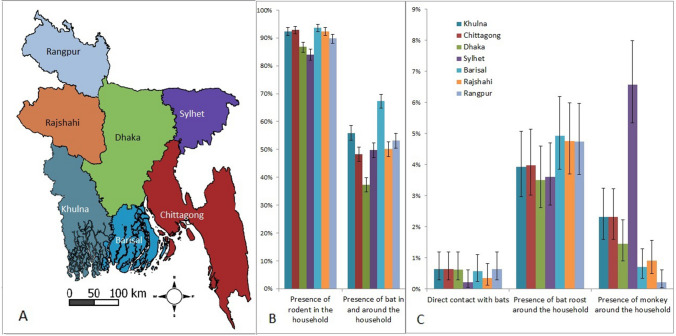
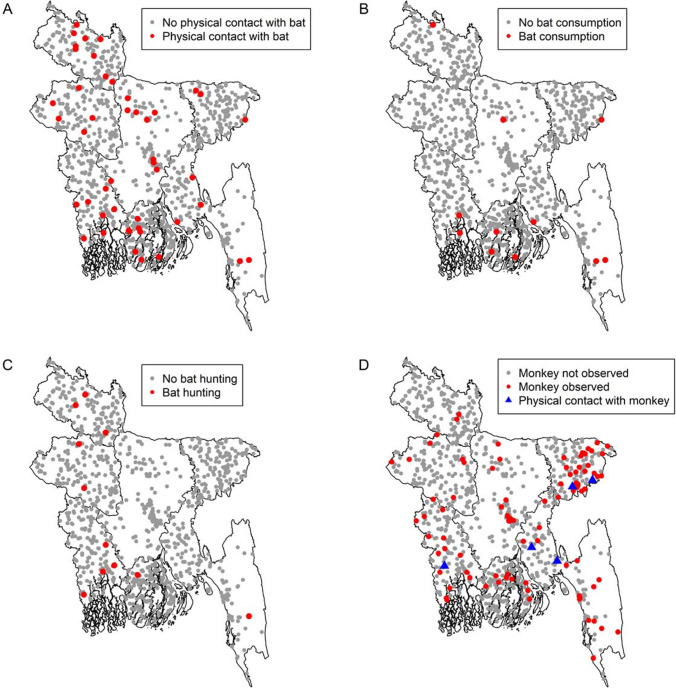
Bat roosts were observed close to the household in 4% of enrolled households (415/10,002; 95% CI 3.7–4.6) and this frequency did not differ by division (Fig. 1B). Bat roosts were reported in 21% of communities (Fig. 2). The presence of bats in or around the household was reported in almost half (52%) of the households in the month prior to the survey. This exposure was significantly highest (67%) (p < 0.001) in Barisal division and lowest in Dhaka division (37%) (p < 0.01) among all divisions (Fig. 1A, B). Most (98%) rural communities reported the presence of bats in the community, but physical contact with bats in the past month was reported in only 0.5% (52/10,002; 95% CI 0.4–0.7) of households (Table 1). Communities reporting direct contact with bats were scattered throughout the country (Fig. 3:A), whereas communities reporting consuming bats were primarily in the south (Fig. 3:B), and communities reporting hunting bats were primarily in the northwestern and southwestern parts of the country (Fig. 3:C).
Figure 1.
A Map of seven divisions of Bangladesh. B Percentage of households (N = 10,002) reporting presence of rodents and bats in and around the households and C direct contact with bats, presence of bat roosts and monkeys around the households across the seven divisions in Bangladesh. Error bars show 95% confidence intervals.
Figure 2.
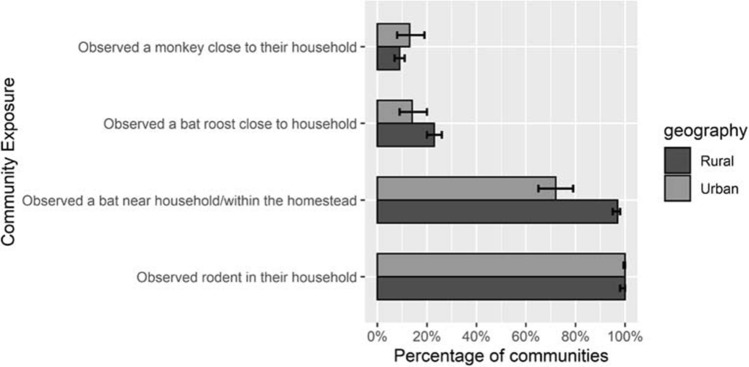
Percentage of communities (N = 1001) reporting presence of bats, rodents and monkeys in their communities. Error bars show 95% confidence intervals.
Figure 3.
Spatial distributions of communities in Bangladesh reporting physical contact with a bat (A), bat consumption (B), bat hunting (C) and presence of monkeys around the households and physical contact with monkeys (D), (N = 1001).
Date Palm Sap Consumption
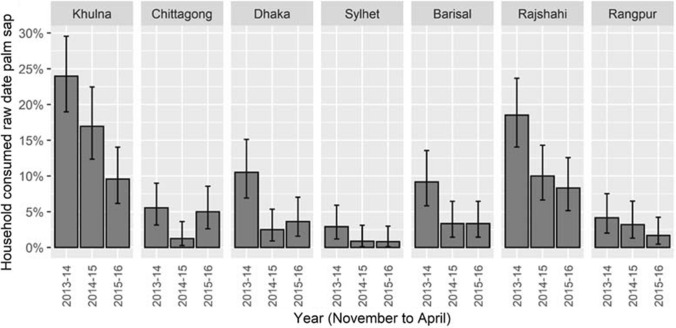
Throughout the country, 7% of respondents (7.5% in rural areas and 5% in urban areas) reported that at least one household member consumed date palm sap during date palm sap season. Between November and April, 1–16% of respondents reported that at least one household member consumed raw date palm sap, whereas less than 0.5% reported drinking sap in other months. Date palm sap consumption was highest during January (16%), followed by February (12%) (Figure S1). The overall percentage of households reporting that a member drank raw date palm sap decreased countrywide (and in most divisions) over the three years of the study, from 11% during 2013–14; 5.5% during 2014–15; and 4.6% during 2015–16 (p < 0.001) (Fig. 4).
Figure 4.
Proportions and 95% confidence intervals of households reporting raw date palm sap consumption during November to April by year of the study and by division in Bangladesh; N = 5801.
The frequency of reporting date palm sap consumption varied among divisions (Fig. 4) from 17% (95% CI 15–20) of households in Khulna, 13%, (95% CI 10–15) in Rajshahi, 5.6% (95% CI 4–8) in Dhaka, 5% (95% CI 4–7) in Barisal, 4% (95% CI 3–6) in Chittagong, 3% (95% CI 2–6) in Rangpur and 1.5% (95% CI 1–3) in Sylhet during date palm consumption seasons during 2013–2016. It was higher in Khulna (p < 0.001 using z-test) and Rajshahi (p < 0.001 using z-test) compared to other divisions.
Presence of Rodents
All 1001 communities and most of the households (90%) throughout the country reported the presence of rodents in their households during the past month (Fig. 1, Table 1). The presence of rodents in the households was similar across divisions (84–94%) (Fig. 1B). A total of 9% of households reported that at least one resident had direct contact with rodents in the past month (Table 1).
Presence of Monkeys
Households in 94 (9.4%) communities reported the presence of monkeys and there were no differences between rural and urban areas (Fig. 2). Overall, 2% (95% CI 1.8–2.4) of households reported the presence of monkeys around their households. The presence of monkeys around the household was higher in the Sylhet division (7%) compared to each of the other divisions (0–2%) (Fig. 1C and Fig. 3:3D). Respondents from 5 communities reported that at least one household member had direct contact with monkeys in the prior month (Fig. 3:3D).
Seasonal Variation of Bats, Rodents and Monkey Exposures
There was no significant seasonal variation in the presence of rodents, bats or monkeys in the study communities (Figure S2). However, raw date palm sap consumption in sampled communities was much more frequent in the winter season (40%, 95% CI 34–45) than in summer (8.6%, 95% CI 6–12) and rainy (1%, CI: 0.2–3%) seasons.
Discussion
Contact with bats, monkeys and rodents was reported throughout the country and in every season, resulting in significant exposures and opportunities for pathogen spillover in Bangladesh. Generalizing the exposure percentages to the entire 160 million people of Bangladesh (World Bank, 2020), every month approximately 13.6 million people have direct contact with rodents, 800,000 have contact with bats and 80,000 with monkeys. Although exposures to these animals were common, there were regional and seasonal patterns that could help inform future studies to identify and investigate the cross-species transmission of infections. For example, monkey exposures were more common in Sylhet division (northeastern Bangladesh) relative to other divisions, suggesting that future work to identify cross-species transmission might focus in this part of the country. Prior studies have highlighted that date palm sap consumption is highest in the winter season, but our study revealed that one of the highest sap consumption regions of Bangladesh is Kushtia, where very few human spillover cases have been detected. It is possible that the mismatch between sap consumption and human infections in that area is due to surveillance gaps (Hegde et al. 2019) and our study shows that surveillance expansion into this area should be considered. Rodent contact was ubiquitous; however, human risk of infection is likely related to the species of rodents in the area, which was impossible to assess with our survey and should be a focus of future work.
Bangladesh hosts 31 bat species including four horseshoe bat species, which are known to carry coronaviruses (Khan 2001), and Pteropus medius fruit bats which host many viruses, including Nipah virus (Anthony et al. 2013). In our survey, 52% of households observed bats, which was similar to another study conducted from 2011 to 2013 (Openshaw et al. 2017). Although the frequency of direct contact with bats was low in both studies, bats and humans share the same environments, and unobserved interactions may occur (Hoyt et al. 2018), including through consumption of date palm sap. Importantly, despite the fact that bats were widely observed across the country, spillovers of Nipah virus are consistently linked with human consumption of date palm sap, rather than observations of bats. Many risk mapping approaches to identify hotspots of emerging infectious diseases rely on species presence maps to define areas of human risk (add refs if you can), but mere spatial overlap many not be insufficient for predicting human risk when there is a particular type of animal-human contact that drives transmission.
This is the first study we know of to investigate the interannual variation in date palm consumption, and it declined nationwide over the three years of our study, from 11% during 2013–2014 to 4.6% during 2015–2016. The reasons for the decreasing consumption were unclear and could possibly be due to interventions or reduced sap availability. The Government of Bangladesh has advised people to avoid raw date palm sap consumption since 2011 to prevent Nipah virus spillovers (The New York Times, 2011), and an intervention trial was conducted in 2012–2014 to promote the use of barrier methods to keep bats from contaminating sap (Nahar et al 2017). However, government messages have had limited distribution and given the timing of the interventions relative to this study, it is unlikely that interventions explain the reduction we observed. A reduction in the quantity or quality of sap produced by date palm trees could also have contributed to lower sap consumption. Cooler temperatures result in sweeter and clearer higher quality sap (Annett et al. 1913; Nahar et al. 2010), and winter temperatures in Bangladesh were relatively warm during the years of our study (2013 and 2016) (McKee et al. 2021). This highlights a potential relationship between Nipah virus transmission risk and weather patterns which warrants further study. Continued monitoring of sap consumption could guide interventions to further reduce risk, including targeting interventions toward those areas with highest consumption.
Rodents have been recognized as hosts of almost 60 zoonotic diseases that pose a serious threat to human health (Meerburg et al. 2009). Rodents were consistently observed in most households (90%) throughout the country over the years of the study, although we were unable to determine specific species from participant reports. The most common rodents near areas of human habitation in Bangladesh are Rattus rattus (house rat), Mus musculus (house mouse), Bandicota indica (greater bandicoot) and B. bengalensis (lesser bandicoot) (Aziz 2011; Sarker et al. 2013). Future work to identify circulating pathogens among these specific rodent species in Bangladesh will help to identify which human-rodent exposures might be most important to target for reducing zoonotic spillover risk.
Further exploration of human exposure to monkeys is recommended in Sylhet division where households with exposure to monkeys were most commonly reported. One study reported the identification of 184 unique viruses from rhesus macaques’ feces including the detection of 8 human viruses, providing direct evidence of contact between humans and these animals (Anthony et al. 2015). Transmission of SFV to a human from a free-ranging population of monkeys was reported in Indonesia in 2000 and in Bangladesh in 2013 (Jones-Engel et al. 2005; Engel et al. 2013). SFV ribonucleic acid (RNA) was found at high concentrations in the oral mucosa and saliva of infected monkeys so primate to human transmission of SFV might occur through bites (Murray et al. 2006). Another pathogen that might spillover from primates to humans is a group A rotavirus (RVA) that was detected by RT-PCR in 4% of rhesus macaques (Macaca mulatta) (N = 445) in Bangladesh (Islam et al. 2020).
Our study has some limitations. We asked participants about their or any of their household members’ exposures to rodents, bats and monkeys within the past month. It is possible that we have underestimated exposures, particularly if respondents were not aware of all members’ exposures. This also means that we do not have individual level information about which household residents had which exposures, precluding the ability to identify individual level risk factors for exposure. We chose a one-month window in an attempt to balance the needs for a reasonable recall period and a long enough recall period to capture relatively infrequent exposures. However, this window may not have been long enough to detect infrequent exposures that might have high transmission rates of pathogens, such as monkey bites (Engel et al. 2013).
Diseases known to be transmitted at the human-wildlife interface could be prevented by targeting high-contact areas for surveillance and prevention efforts. Our study has highlighted the ubiquity of rodent exposure in Bangladesh, areas of high human consumption of date palm sap that could benefit from Nipah virus surveillance efforts, and the relatively infrequent but geographically clustered direct contact with monkeys that could be used to advance public health research on zoonoses and surveillance. Remaining questions about interannual sap consumption patterns, and the specific species of rodents responsible for human exposures and the pathogens they carry warrant additional study. Our approach could be useful for other countries aiming to better understand spatial and temporal variation in animal-human contact and could be used to better inform mapping risk of spillovers.
Supplementary Information
Below is the link to the electronic supplementary material.
Figure S1. Proportion and 95% confidence interval of households reporting drinking raw date palm sap, by month, Bangladesh (N = 10,002) (TIFF 23337 KB)
Figure S2. Proportion and 95% confidence interval of communities reporting presence of bats, monkeys and rodents (N: 1001) by season. Summer season includes March–June, rainy includes July–October, and winter includes November–February (TIFF 17845 KB)
Acknowledgements
We are grateful to our study participants for their time and valuable information. We thank Md Mahabub Hasan Chowdhury, Md. Asaduzzaman, Ashok Kumar Biswas, Md Rashedul Azim, Ashish Kumar Kundu, S.A.M. Safiur Rahman, Parvin Akter Tithe, S.M. Hasan Al-Mahmud, Md. Tarequl Islam and Md. Masudur Rahman for their valuable contribution in data collection. We also thank Gladys Leterme, icddr,b for her constructive support in editing this manuscript. We acknowledge the Centers for Disease Control and Prevention, Atlanta, GA, USA, for funding (CoAg Grant 5-U01GH001207). We are also grateful to icddr,b and its core donors, the Governments of Bangladesh, Canada, Sweden and the UK, for supporting this work.
Funding
The Centers for Disease Control and Prevention, Atlanta, GA, USA, CoAg Grant 5-U01GH001207
Declarations
Ethical approval
The protocol was reviewed and approved by icddr,b’s research review and ethical review committees (Protocol number # PR-13033). The U.S. Centers for Disease Control and Prevention relied on icddr,b’s determination.
Informed consent
We obtained written, informed consent from each respondent before administering the questionnaire.
References
- Alimohamadi Y, Sepandi M. Considering the design effect in cluster sampling. Journal of Cardiovascular and Thoracic Research. 2019;11:78–78. doi: 10.15171/jcvtr.2019.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annett HE, Amin BM, Lele GK (1913) The date sugar industry in Bengal: an investigation into its chemistry and agriculture. Imperial Department of Agriculture in India
- Anthony SJ, Epstein JH, Murray KA, Navarrete-Macias I, Zambrana-Torrelio CM, Solovyov A, Ojeda-Flores R, Arrigo NC, Islam A, Ali Khan S. A strategy to estimate unknown viral diversity in mammals. Mbio. 2013;4:e00598–e613. doi: 10.1128/mBio.00598-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony SJ, Islam A, Johnson C, Navarrete-Macias I, Liang E, Jain K, Hitchens PL, Che X, Soloyvov A, Hicks AL. Non-random patterns in viral diversity. Nature Communications. 2015;6:1–7. doi: 10.1038/ncomms9147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arya SC (1994) Plague in India. BMJ: British Medical Journal 309:1369 [DOI] [PMC free article] [PubMed]
- Aziz MA (2011) Notes on the status of mammalian fauna of the Lawachara National Park, Bangladesh. Ecoprint: An International Journal of Ecology 18:45–53
- Bangladesh Bureau of Statistics (2015) Trends, patterns and determinants of marriage in Bangladesh. Report number: 13
- Bhardwaj P, Kosambiya JK, Desai VK (2008) A case control study to explore the risk factors for acquisition of leptospirosis in Surat city, after flood. Indian journal of medical sciences 62 [PubMed]
- Biswas S. Plague in India: A review. Journal of Communicable Diseases. 2018;50:60–75. doi: 10.24321/0019.5138.201821. [DOI] [Google Scholar]
- Butler T. Plague into the 21st century. Clinical Infectious Diseases. 2009;49:736–742. doi: 10.1086/604718. [DOI] [PubMed] [Google Scholar]
- Calisher CH, Childs JE, Field HE, Holmes KV, Schountz T. Bats: important reservoir hosts of emerging viruses. Clinical Microbiology Reviews. 2006;19:531–545. doi: 10.1128/CMR.00017-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC (2017) Diseases directly transmitted by rodents Available: https://www.cdc.gov/rodents/diseases/direct.html [accessed February 10, 2022]
- Chandy S, Yoshimatsu K, Ulrich RG, Mertens M, Okumura M, Rajendran P, John GT, Balraj V, Muliyil J, Mammen J, Abraham P. Seroepidemiological study on hantavirus infections in India. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2008;102(1):70–74. doi: 10.1016/j.trstmh.2007.09.016. [DOI] [PubMed] [Google Scholar]
- Engel GA, Small CT, Soliven K, Feeroz MM, Wang X, Kamrul Hasan M, Oh G, RabiulAlam SM, Craig KL, Jackson DL. Zoonotic simian foamy virus in Bangladesh reflects diverse patterns of transmission and co-infection. Emerging Microbes & Infections. 2013;2:1–10. doi: 10.1038/emi.2013.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein JH, Anthony SJ, Islam A, Kilpatrick AM, Ali Khan S, Balkey MD, Ross N, Smith I, Zambrana-Torrelio C, Tao Y. Nipah virus dynamics in bats and implications for spillover to humans. Proceedings of the National Academy of Sciences. 2020;117:29190–29201. doi: 10.1073/pnas.2000429117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Zhao K, Shi Z-L, Zhou P. Bat Coronaviruses in China. Viruses. 2019;11:210. doi: 10.3390/v11030210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feeroz MM, Soliven K, Small CT, Engel GA, Andreina Pacheco M, Yee JL, Wang X, Kamrul Hasan M, Oh G, Levine KL. Population dynamics of rhesus macaques and associated foamy virus in Bangladesh. Emerging Microbes & Infections. 2013;2:1–14. doi: 10.1038/emi.2013.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurley ES, Hegde ST, Hossain K, Sazzad HM, Hossain MJ, Rahman M, Sharker MY, Salje H, Islam MS, Epstein JH. Convergence of humans, bats, trees, and culture in Nipah virus transmission. Bangladesh. Emerging Infectious Diseases. 2017;23:1446. doi: 10.3201/eid2309.161922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurley ES, Montgomery JM, Hossain MJ, Bell M, Azad AK, Islam MR, Molla MAR, Carroll DS, Ksiazek TG, Rota PA. Person-to-person transmission of Nipah virus in a Bangladeshi community. Emerging Infectious Diseases. 2007;13:1031. doi: 10.3201/eid1307.061128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurley ES, Spiropoulou CF, De Wit E. Twenty years of Nipah virus research: where do we go from here? The Journal of Infectious Diseases. 2020;221:S359–S362. doi: 10.1093/infdis/jiaa078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider N, Rahman MS, Khan SU, Mikolon A, Gurley ES, Osmani MG, Shanta IS, Paul SK, Macfarlane-Berry L, Islam A. Identification and Epidemiology of a Rare HoBi-Like Pestivirus Strain in B angladesh. Transboundary and Emerging Diseases. 2014;61:193–198. doi: 10.1111/tbed.12218. [DOI] [PubMed] [Google Scholar]
- Hasan MK, Aziz MA, Alam SMR, Kawamoto Y, Jones-Engel L, Kyes RC, Akhtar S, Begum S. Feeroz MM (2013) Distribution of Rhesus Macaques (Macaca mulatta) in Bangladesh: Inter-population Variation in Group Size and Composition. Primate Conservation. 2013;26:125–132. doi: 10.1896/052.026.0103. [DOI] [Google Scholar]
- Hegde ST, Salje H, Sazzad HM, Hossain MJ, Rahman M, Daszak P, Klena JD, Nichol ST, Gurley Luby SP, ES, Using healthcare-seeking behaviour to estimate the number of Nipah outbreaks missed by hospital-based surveillance in Bangladesh. International Journal of Epidemiology. 2019;48(4):1219–1227. doi: 10.1093/ije/dyz057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde ST, Sazzad HM, Hossain MJ, Alam MU, Kenah E, Daszak P, Rollin P, Rahman M, Luby SP, Gurley ES. Investigating Rare Risk Factors for Nipah Virus in Bangladesh: 2001–2012. Ecohealth. 2016 Dec;13(4):720–728. doi: 10.1007/s10393-016-1166-0. Epub 2016 Oct 13. PMID: 27738775; PMCID: PMC5164848. [DOI] [PMC free article] [PubMed]
- Holmes EC, Goldstein SA, Rasmussen AL, Robertson DL, Crits-Christoph A, Wertheim JO, Anthony SJ, Barclay WS, Boni MF, Doherty PC. The origins of SARS-CoV-2: A critical review. Cell. 2021;184:4848–4856. doi: 10.1016/j.cell.2021.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homaira N, Rahman M, Hossain MJ, Epstein JH, Sultana R, Khan MSU, Podder G, Nahar K, Ahmed B, Gurley ES. Nipah virus outbreak with person-to-person transmission in a district of Bangladesh, 2007. Epidemiology & Infection. 2010;138:1630–1636. doi: 10.1017/S0950268810000695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyt JR, Langwig KE, White JP, Kaarakka HM, Redell JA, Kurta A, DePue JE, Scullon WH, Parise KL, Foster JT, Frick WF, Kilpatrick AM. Cryptic connections illuminate pathogen transmission within community networks. Nature. 2018;563:710–713. doi: 10.1038/s41586-018-0720-z. [DOI] [PubMed] [Google Scholar]
- IEDCR (2021) Yearly distribution of Nipah cases in Bangladesh 2001–2021 Available:https://iedcr.gov.bd/surveillances/93c87e70-9c22-4f21-9506-a1161ecf404f [Accessed 5 December 2021]
- Islam A, Hossain ME, Haider N, Rostal MK, Mukharjee SK, Ferdous J, Miah M, Rahman M, Daszak P, Rahman MZ. Molecular characterization of group A rotavirus from rhesus macaques (Macaca mulatta) at human–wildlife interfaces in Bangladesh. Transboundary and Emerging Diseases. 2020;67:956–966. doi: 10.1111/tbed.13431. [DOI] [PubMed] [Google Scholar]
- Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, Daszak P. Global trends in emerging infectious diseases. Nature. 2008;451:990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones-Engel L, Engel GA, Schillaci MA, Rompis A, Putra A, Suaryana KG, Fuentes A, Beer B, Hicks S, White R. Primate-to-human retroviral transmission in Asia. Emerging Infectious Diseases. 2005;11:1028. doi: 10.3201/eid1107.040957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabir MI, Rahman MB, Smith W, Lusha MAF, Milton AH. Climate change and health in Bangladesh: a baseline cross-sectional survey. Global Health Action. 2016;9:29609. doi: 10.3402/gha.v9.29609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser R, Woodruff BA, Bilukha O, Spiegel PB, Salama P. Using design effects from previous cluster surveys to guide sample size calculation in emergency settings. Disasters. 2006;30:199–211. doi: 10.1111/j.0361-3666.2006.00315.x. [DOI] [PubMed] [Google Scholar]
- Kendall EA, LaRocque RC, Bui DM, Galloway R, Ari MD, Goswami D, Breiman RF, Luby S, Brooks WA. Leptospirosis as a cause of fever in urban Bangladesh. The American Journal of Tropical Medicine and Hygiene. 2010;82:1127. doi: 10.4269/ajtmh.2010.09-0574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MAR. Status and distribution of bats in Bangladesh with notes on their ecology. Zoos’ Print Journal. 2001;16:479–483. doi: 10.11609/JoTT.ZPJ.16.5.479-83. [DOI] [Google Scholar]
- Khan M (2008) Protected Areas of bangladesh; A guide to wildlife. Nishorgo Program, Wildlife Management and Nature Conservation Circle, Bangladesh Forest Department; 61–63
- Leroy EM, Epelboin A, Mondonge V, Pourrut X, Gonzalez J-P, Muyembe-Tamfum J-J, Formenty P. Human Ebola outbreak resulting from direct exposure to fruit bats in Luebo, Democratic Republic of Congo, 2007. Vector-Borne and Zoonotic Diseases. 2009;9:723–728. doi: 10.1089/vbz.2008.0167. [DOI] [PubMed] [Google Scholar]
- Luby SP, Rahman M, Hossain MJ, Blum LS, Husain MM, Gurley E, Khan R, Ahmed B-N, Rahman S, Nahar N. Foodborne transmission of Nipah virus, Bangladesh. Emerging Infectious Diseases. 2006;12:1888. doi: 10.3201/eid1212.060732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby SP. The pandemic potential of Nipah virus. Antiviral Res. 2013;100(1):38–43. doi: 10.1016/j.antiviral.2013.07.011. [DOI] [PubMed] [Google Scholar]
- McKee CD, Islam A, Luby SP, Salje H, Hudson PJ, Plowright RK, Gurley ES. The ecology of Nipah virus in Bangladesh: a nexus of land-use change and opportunistic feeding behavior in bats. Viruses. 2021;13:169. doi: 10.3390/v13020169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerburg BG, Singleton GR, Kijlstra A. Rodent-borne diseases and their risks for public health. Critical Reviews in Microbiology. 2009;35:221–270. doi: 10.1080/10408410902989837. [DOI] [PubMed] [Google Scholar]
- Moniruzzaman S. Crop choice as climate change adaptation: Evidence from Bangladesh. Ecological Economics. 2015;118:90–98. doi: 10.1016/j.ecolecon.2015.07.012. [DOI] [Google Scholar]
- Murray SM, Picker LJ, Axthelm MK, Linial ML. Expanded tissue targets for foamy virus replication with simian immunodeficiency virus-induced immunosuppression. Journal of Virology. 2006;80:663–670. doi: 10.1128/JVI.80.2.663-670.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahar N, Asaduzzaman M, Mandal UK, Rimi NA, Gurley ES, Rahman M, Garcia F, Zimicki S, Sultana R, Luby SP. Hunting bats for human consumption in Bangladesh. EcoHealth. 2020;17:139–151. doi: 10.1007/s10393-020-01468-x. [DOI] [PubMed] [Google Scholar]
- Nahar N, Paul RC, Sultana R, Sumon SA, Banik KC, Abedin J, Asaduzzaman M, Garcia F, Zimicki S, Rahman M. A controlled trial to reduce the risk of human Nipah virus exposure in Bangladesh. Ecohealth. 2017;14:501–517. doi: 10.1007/s10393-017-1267-4. [DOI] [PubMed] [Google Scholar]
- Nahar N, Sultana R, Gurley ES, Hossain MJ, Luby SP. Date palm sap collection: exploring opportunities to prevent Nipah transmission. EcoHealth. 2010;7:196–203. doi: 10.1007/s10393-010-0320-3. [DOI] [PubMed] [Google Scholar]
- Nikolay B, Salje H, Hossain MJ, Khan AD, Sazzad HM, Rahman M, Daszak P, Ströher U, Pulliam JR, Kilpatrick AM. Transmission of Nipah virus—14 years of investigations in Bangladesh. New England Journal of Medicine. 2019;380:1804–1814. doi: 10.1056/NEJMoa1805376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Openshaw JJ, Hegde S, Sazzad HM, Khan SU, Hossain MJ, Epstein JH, Daszak P, Gurley ES, Luby SP. Bat hunting and bat–human interactions in Bangladeshi villages: Implications for zoonotic disease transmission and bat conservation. Transboundary and Emerging Diseases. 2017;64:1287–1293. doi: 10.1111/tbed.12505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plowright RK, Parrish CR, McCallum H, Hudson PJ, Ko AI, Graham AL, Lloyd-Smith JO. Pathways to zoonotic spillover. Nature Reviews Microbiology. 2017;15:502–510. doi: 10.1038/nrmicro.2017.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu X, Wong G, Audet J, Bello A, Fernando L, Alimonti JB, Fausther-Bovendo H, Wei H, Aviles J, Hiatt E. Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature. 2014;514:47–53. doi: 10.1038/nature13777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman MA, Hossain MJ, Sultana S, Homaira N, Khan SU, Rahman M, Gurley ES, Rollin PE, Lo MK, Comer JA. Date palm sap linked to Nipah virus outbreak in Bangladesh, 2008. Vector-Borne and Zoonotic Diseases. 2012;12:65–72. doi: 10.1089/vbz.2011.0656. [DOI] [PubMed] [Google Scholar]
- Salah Uddin Khan M, Hossain J, Gurley ES, Nahar N, Sultana R, Luby SP. Use of infrared camera to understand bats’ access to date palm sap: implications for preventing Nipah virus transmission. Ecohealth. 2010;7:517–525. doi: 10.1007/s10393-010-0366-2. [DOI] [PubMed] [Google Scholar]
- Sarker NJ, Rokunuzzam MD, Nessa RR. Abundance of rats and mice in the selected areas of Dhaka city: A cross sectional study. Journal of Entomology and Zoology Studies. 2013;1:116–119. [Google Scholar]
- Sazzad HM, Hossain MJ, Gurley ES, Ameen KM, Parveen S, Islam MS, Faruque LI, Podder G, Banu SS, Lo MK. Nipah virus infection outbreak with nosocomial and corpse-to-human transmission. Bangladesh. Emerging Infectious Diseases. 2013;19:210. doi: 10.3201/eid1902.120971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shano S, Islam A, Hagan E, Rostal MK, Martinez S, Al Shakil A, Hasan M, Francisco L, Husain MM, Rahman M. Environmental Change and Zoonotic Disease Risk at Human-Macaque Interfaces in Bangladesh. EcoHealth. 2021;18:487–499. doi: 10.1007/s10393-021-01565-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- South Asia Environment Outlook (2009) United Nations Environment Programme; 2009. Available:www.saarc-sec.org/userfiles/SAEO%202009.pdf [accessed February 10, 2022]
- The Daily Star (2014) Primates of Bangladesh. Available: https://www.thedailystar.net/primates-of-bangladesh-14611 [Accessed 5 June 2018].
- The Daily Star (2017) Date palm juice, patali gur on decline Available:https://www.thedailystar.net/country/date-palm-juice-patali-gur-decline-1343920 [Accessed 2020 Sep 13]
- The New York Times (2011) Bangladesh Bans Sale of Palm Sap After an Unusually Lethal Oubreak available:https://www.nytimes.com/2011/03/22/health/22global.html [Accessed 2020 Sep 10]
- World Bank (2017) Population density (people per sq. km of land area) | Data Available:http://data.worldbank.org/indicator/EN.POP.DNST [accessed February 10, 2022]
- World Bank (2020) Population, total - Bangladesh | Data Available:https://data.worldbank.org/indicator/SP.POP.TOTL?locations=BD [Accessed November 1, 2020].
- Wolfe ND, Dunavan CP, Diamond J. Origins of major human infectious diseases. Nature. 2007;447:279–283. doi: 10.1038/nature05775. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Proportion and 95% confidence interval of households reporting drinking raw date palm sap, by month, Bangladesh (N = 10,002) (TIFF 23337 KB)
Figure S2. Proportion and 95% confidence interval of communities reporting presence of bats, monkeys and rodents (N: 1001) by season. Summer season includes March–June, rainy includes July–October, and winter includes November–February (TIFF 17845 KB)