Figure 1.
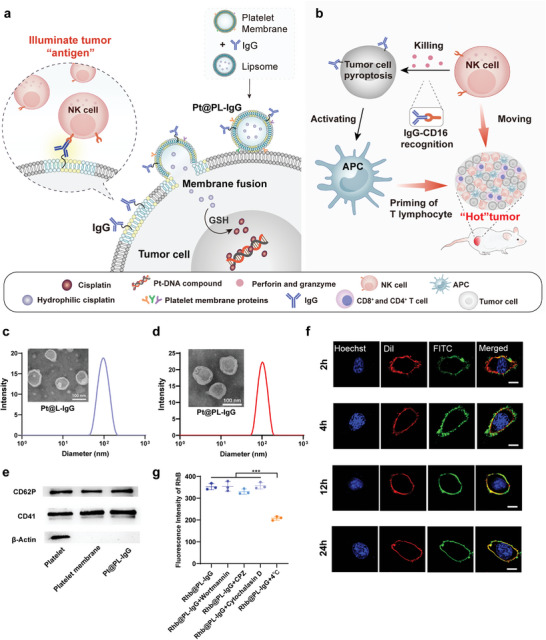
Design of the illuminate tumor homogenization antigen properties (ITHAP) strategy and characterization of Pt@PL‐IgG. a) The process of bio‐liposomes interaction with tumor cells. Following intravenous administration, Pt@PL‐IgG was recognized and adhered to the tumor cells by the platelet membranes, and fused with the tumor cells to insert NK‐activatable target antigen “IgG” onto the surface of the tumor cells. b) Following implantation of the new antigen, the tumor cells were identified by NK cells and triggered the ADCC effect. Meanwhile, the killed tumor cells activated APCs, which worked in concert with NK cells to alleviate the immunosuppressed tumor microenvironment and shape the “hot” tumor. c) Histogram of the size distribution and representative TEM images of Pt@L‐IgG. d) Histogram of the size distribution and representative TEM images of Pt@PL‐IgG. e) The expression of proteins of platelets on Pt@PL‐IgG. f) Fusogenic property of Pt@PL‐FITC evaluated using CLSM. Phospholipids were labeled with FITC (green), tumor cell membrane was labeled with DiI (red), and tumor cell nucleus was labeled with Hoechst (blue). Scale bar, 5 µm. g) Fluorescence intensity of rhodamine b (RhB) in 4T1 cells treated with PBS, wortmannin, chlorpromazine, and cytochalasin D at 37 °C and PBS at 4 °C followed by incubation with Rhb@PL‐IgG in vitro by flow cytometry, respectively (n = 3). Data are shown as mean ± SD; n represents the number of biologically independent samples. Student's t‐test. *P < 0.05, **P < 0.01 and ***P < 0.001 and ****P < 0.0001.
