Abstract
Radiofrequency (RF) catheter ablation has become a widely used therapeutic approach. However, long-term results in terms of arrhythmia recurrence are still suboptimal. Cardiac magnetic resonance (CMR) could offer a valuable tool to overcome this limitation, with the possibility of targeting the arrhythmic substrate and evaluating the location, depth, and possible gaps of RF lesions. Moreover, real-time CMR-guided procedures offer a radiation-free approach with an evaluation of anatomical structures, substrates, RF lesions, and possible complications during a single procedure. The first steps in the field have been made with cavotricuspid isthmus ablation, showing similar procedural duration and success rate to standard fluoroscopy-guided procedures, while allowing visualization of anatomic structures and RF lesions. These promising results open the path for further studies in the context of more complex arrhythmias, like atrial fibrillation and ventricular tachycardias. Of note, setting up an interventional CMR (iCMR) centre requires safety and technical standards, mostly related to the need for CMR-compatible equipment and medical staff’s educational training. For the cardiac imagers, it is fundamental to provide correct CMR sequences for catheter tracking and guide RF delivery. At the same time, the electrophysiologist needs a rapid interpretation of CMR images during the procedures. The aim of this paper is first to review the logistic and technical aspects of setting up an iCMR suite. Then, we will describe the experience in iCMR-guided flutter ablations of two European centres, Policlinico Casilino in Rome, Italy, and Haga Teaching Hospital in The Hague, the Netherlands.
Keywords: Interventional CMR, Cardiac magnetic resonance, Magnetic resonance imaging, Electrophysiology, Flutter, Arrhythmias
Introduction
Atrial arrhythmias, and more specifically atrial fibrillation (AF) and atrial flutter (AFL), represent a major health burden, as they are associated with an increased risk of stroke and heart failure, and decreased quality of life. Current guidelines1 suggest catheter ablation as a valuable therapeutic strategy for treating supraventricular tachycardias.
Nonetheless, arrhythmia recurrence following an initially successful procedure is still higher than expected.2 This happens despite the extensive use of three-dimensional (3D) mapping systems that create an anatomical representation of the cardiac chambers according to voltage, magnetic and impedance measurements. Moreover, current catheter ablation is not able to real-time visualize ablation lesions, precluding further assessment of ablation success and quality.
Cardiac magnetic resonance (CMR) has emerged over the past few years as an interesting and promising method to guide ablations. From a very early stage of procedural planning, CMR is useful in providing anatomical information and tissue characterization with a single technique. Indeed, high-contrast and high-resolution CMR images allow a complete delineation of the patient’s atrial anatomy, including detection of possible anatomical variation, rule out of thrombosis, and visualization of left atrial oedema and fibrosis3
Even though CMR-guided ablations could be particularly suitable for treating complex arrhythmias such as ventricular tachycardias, treating relatively ‘simple’ cardiac arrhythmias using a real-time interventional CMR (iCMR) guide seems to be the fundamental first step to gaining experience with this new approach. In particular, ablation of the cavotricuspid isthmus (CTI) by iCMR real-time guidance is increasingly performed in different centres with performance and outcome measures similar to conventional, fluoroscopy-guided flutter ablation.4
In this paper, we will first review the technical aspects of iCMR-guided AFL ablation, both from the cardiac imagers’ and electrophysiologists’ points of view. Then, we will describe the experience of two European centres in iCMR-guided AFL ablation, Policlinico Casilino in Rome, Italy, and Hagaziekenhuis teaching hospital, in the Hague, the Netherlands.
CMR-guided ablation of atrial flutter
In 2019, Paetsch et al.5 demonstrated the safety and success in a routine clinical setting of real-time CMR-guided CTI ablation in AFL. The primary success rate of iCMR-guided ablation was 93%, and none of the 30 patients enrolled had clinical or documented episodes of AFL recurrence over a follow-up period of 3 months.5 Compared with the control group, who underwent a conventional fluoroscopy-guided CTI ablation procedure, the safety and efficacy of iCMR and of conventional fluoroscopy-guided CTI ablation procedures were similar.5 The duration of the two different techniques was almost identical.5
Apart from being radiation-free, the advantages of iCMR for CTI ablation consist in real-time tracking of the catheters with visualization of catheter-tissue contact in the target region, direct visualization of acute RF-induced lesions (in terms of presence, localization, and extent of right atrial oedema or late gadolinium enhancement—LGE) and detection of possible complications such as cardiac tamponade. Moreover, detailed imaging of the patient’s anatomy reveal anatomical challenges such as deep pouches or a prominent Eustacian ridge.
The main challenge of iCMR procedures is setting up the iCMR suite for iCMR-guided procedures. This involves procurement of MRI compatible equipment, reimbursement issues, together with need for educational training. As far as the technical aspects are concerned, reliable CMR imaging and tracking of the catheter tip are challenging due to the heart's motion resulting from cardiac and respiratory cycles, especially in patients with arrhythmias or shortness of breath. Moreover, using gradient-echo and balanced steady-state free precession (bSSFP) CMR sequences, only frame rates of 2–8/s can be achieved, limiting the temporal resolution of real-time CMR.4 Finally, cardiac implantable electronic devices may cause off-resonance artefacts that impair image assessment, particularly in anterior and lateral left ventricular wall segments. In general, using CMR guidance introduces a new level of complexity into interventional electrophysiology. In order to obtain successful results, the cardiac imager and the electrophysiologist need to coordinate every step of the procedure. The electrophysiologist must become proficient with catheter manipulation using CMR images only, whereas the CMR expert has to develop an understanding of what the electrophysiologist needs to see in order to successfully navigate bloodvessels and cardiac structures.
Set up of interventional CMR (iCMR) from the cardiac imager point of view: The Hague experience
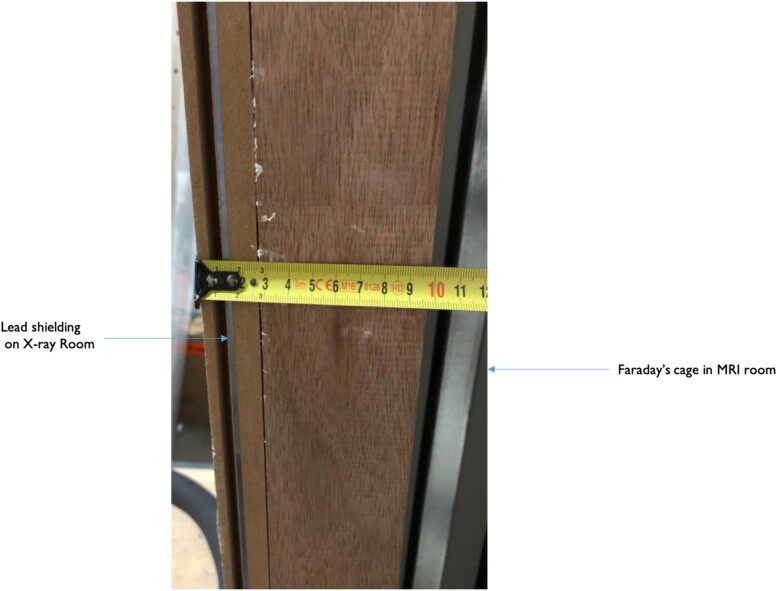
In November 2019, the CMR ablation centre opened in The Haga Teaching Hospital in The Hague, The Netherlands. This is a dedicated interventional CMR suite with fluoroscopy X-ray room for conventional electrophysiological procedures, with a directly connected CMR room (see Figure 1 for the floor plan). The fluoroscopy section is lead shielded, whereas the CMR section is shielded by a Faraday's cage.
Figure 1.
Overview of the interventional cardiovascular magnetic resonance suite. In the iCMR environment, the EP lab and the iCMR room are adjacent to prepare the patient for the CMR-guided procedure and to guarantee safety and prompt intervention in case of complications. Each interventional space has its own control room.
Figure 2 shows the lead shielding and Faraday's cage. In this room, a 1.5 T Siemens CMR scanner (MAGNETOM Aera, Siemens Healthcare, Erlangen, Germany) is installed. This facility is ideally suitable for CMR-guided interventions. Patients’ safety is optimized because of the dedicated facility and the dedicated team consisting of trained and experienced CMR technicians, cath lab nurses, imaging cardiologists, and electrophysiologists, and the equipment is optimized. Additionally, operating room-grade air treatment systems are installed allowing for sterile procedures.
Figure 2.
Lead shielding (on the left side of the wall), separating the electrophysiology lab room from the MRI room, provided of Faraday’s cage (right side of the wall).
The first CMR-guided flutter ablation took place in June 2020, making this the first CMR-guided flutter ablation in the Benelux. This first procedure took 160 min, mostly because of the acquisition of extra CMR sequences. By the time we performed the 5th flutter ablation, the procedure time was shortened to 30 min.
When setting up an iCMR suite, the new environment needs to meet the safety and technical standards for both CMR and surgical standards, especially considering that all standard electrophysiological (EP) and anaesthetic instruments should be replaced with CMR-compatible alternatives. Firstly, performing CMR-guided EP procedures requires CMR-compatible CE-approved EP equipment. In Hagaziekenhuis teaching hospital, ablation catheters (Vision-MR Catheters, IMRICOR Medical Systems, Burnsville, MN) are CMR-conditional, open-irrigated, steerable, with a fibre optic temperature sensing tip and two integrated CMR receive coils for active tracking. They are connected to an EP recorder and stimulator system (Advantage-MR, Medical Systems), which consists of a patient–device interface, a digital amplifier stimulator located in the scanner room, and a host computer placed in the control room. It displays and records both intracardiac and external electrograms, other than performing pacing and tip temperature monitoring. The Advantage-MR system also integrates RF energy for therapy delivery: an RF generator and irrigation in the control room are connected to the ablation catheter through a waveguide for delivery for RF energy and for catheter irrigation. CMR-guided ablation procedures are performed in a conventional 1.5 Tesla magnetic resonance imaging (CMR) unit (MAGNETOM Aera, Siemens Healthcare, Erlangen, Germany). A CMR-conditional patient monitoring system is located in the scanner room and provides real-time patient vitals and electrocardiographic monitoring. Other materials essential for performing iCMR are compatible anaesthetic equipment for patient sedation and MR-compatible headsets for communication between the scanner room and the control room as well as providing hearing protection for the operators. Ferromagnetic instruments that cannot be modified must remain outside the scanner room. As for the RF generator, it is positioned in the control room.
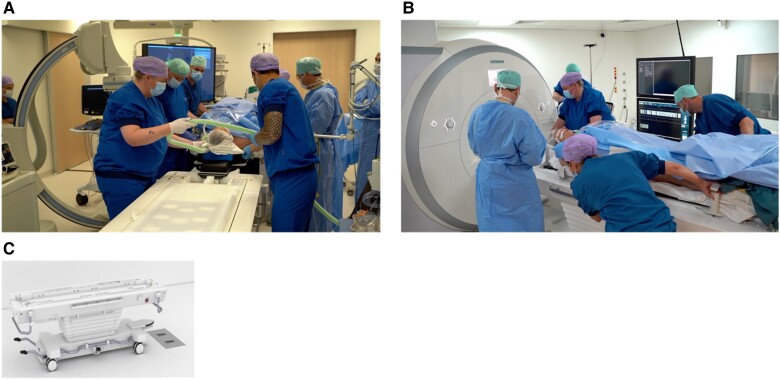
The ideal iCMR procedural workflow includes (i) iCMR suite preparation with the necessary equipment and (ii) patient preparation in the adjacent EP lab, which consists of anaesthesia induction, sterile draping of the patient, and vascular access to the femoral vein. After removal of all MR unsafe material, (iii) the patient is transferred to the iCMR suite (Figure 3), and (iv) the iCMR procedure then started with baseline CMR and insertion of the catheter. After completion of the ablation procedure, (v) CMR is performed to exclude complications and evaluate the produced lesions via tissue characterization. The iCMR spaces and environment are built to provide the realization of the above-explained workflow.
Figure 3.
After performing anaesthesia and vascular access isolation, the patient is moved from the EP room (A) to the scanner room (B) by using a sliding table (C).
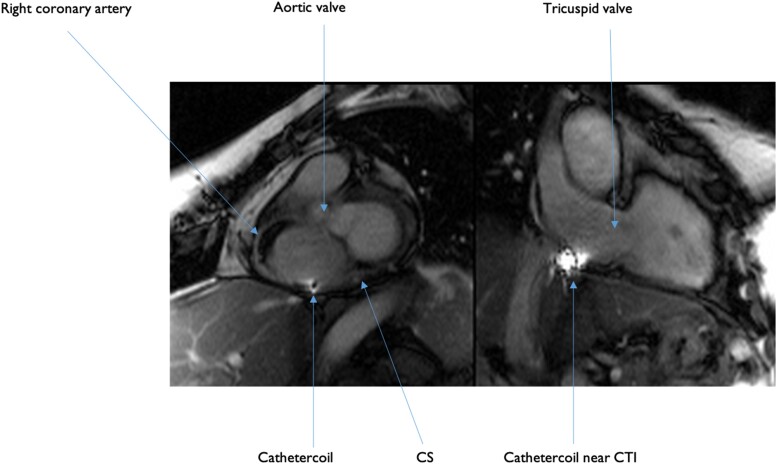
Imaging of CMR-guided CTI ablation starts in the preprocedural phase with an ECG-triggered respiratory-navigated 3D steady-state free precession (SSFP) sequences to acquire a full volume anatomy of the heart and of surrounding structures. Ideally, from this acquisition a 3D shell is obtained and integrated into a navigation system, creating a map for the procedure. However, good electro-anatomical mapping software is unfortunately still under development. The CTI is visualized in different imaging planes, similarly to standard fluoroscopy views depending on the operator’s preference. Another feature is interactive tracking, meaning that the multiplanar reconstruction of the live sequence automatically adjusts to the catheter position. The 3D volume map is later fundamental for active tracking of the catheters during the procedure. Regarding this, a distinction must be made between passive and active catheter tracking. In passive catheter tracking, the catheter is visualized by a signal void in T2-weighted (T2-w) CMR images due to artefacts caused by ferromagnetic substances at the tip of the catheter, or by a hyper-enhanced signal in T1-weighted (T1w) images that is caused by, for instance, gadolinium-based compounds at the tip of the catheter. In contrast, active tracking uses micro coils within the catheter tip to determine its location. A dedicated tracking sequence allows for detection of the catheter position and orientation and to superimpose/integrate this information into the 3D heart-volume acquired before the procedure (Figure 4). Moreover, the catheter tip is automatically aligned to the correct imaging plane during active tracking and the examiner can switch between active catheter tracking and real-time CMR imaging in any moment, to verify the catheter position before delivery of radiofrequency. This is of substantial use since active tracking visualizes the current catheter position in the coordinate system of the CMR scanner and not in a 1:1 relation to the previously acquired 3D volume, meaning that respiratory and/or patient motion can lead to registration errors of the catheter, requiring real-time confirmation scans. Moving on to the post-procedural assessment, CMR is the method of choice for acute lesion assessment with its excellent soft tissue contrast. As far as LGE acquisition is concerned, the lesion size detected by LGE images depends largely on the time interval between gadolinium administration and image acquisition, displaying a smaller lesion size in case of later LGE image acquisition. Moreover, LGE imaging in the acute setting may overestimate the lesion size because oedematous border zones surrounding the lesion core also show presence of LGE, as demonstrated in comparison to LGE images obtained 3 months after the procedure.6 Similarly, T2-w imaging of lesion size based on the detection of hyper-enhanced areas rather overestimates the size of the acute lesion,7 even if it has been successfully applied in patients with atrial flutter who underwent real-time CMR-guided CTI ablation. On the contrary, the lesion size appears to remain the same in native T1w images, supporting the notion that T2-w imaging is more appropriate in detecting myocardial oedema independently of concurrent scarring. A non-contrast-enhanced T1w approach with a 3D-spoiled gradient-echo sequence and a long inversion-time enables visualization of the lesions as a ‘hyperintense’ core signal with a surrounding ‘hypointense’ band of necrosis, oedema, and haematoma. Similarly, chronic scars could be depicted by a ‘hypointense’ signal.8 In conclusion, in comparison to more traditional approaches, non-contrast-enhanced T1w 3D-spoiled gradient-echo sequences may improve detection and differentiation of acute and chronic ablation lesions with further definition of core and border zones.
Figure 4.
CMR still frame image on of IMRICOR’s active tracking catheter. Please note the biplane image which is set on right anterior oblique view (RAO) but which can be freely rotated or set for automatic tracking. Any orientation can be chosen by the operator.
Set up of interventional CMR (iCMR) from the electrophysiologist point of view: the Rome experience
On 28 July 2022, the CMR-Guided Ablation Programme at Policlinico Casilino started. Since then, three patients with AFL procedures underwent an iCMR-guided ablation. All procedures were performed under general anaesthesia. CMR images were obtained with Ingenia Evolution 1,5 T (Philips Healthcare). Patients were intubated outside of the CMR environment. Two 25 cm long sheaths were inserted in the right femoral vein. Two 9 F CMR-compatible ablation catheters were prepared, the first one with full irrigation and the second one just flushed before insertion. Catheters were then advanced through the inferior vena cava into the heart and then positioned into the coronary sinus and the other in the proximity of the CTI. CMR images were able to show the difficult anatomy of one patient with a long isthmus and the presence of a pouch. In our patients, ablation was performed with 4 45W-RF deliveries of 30 s using a Vision-MR IMRICOR catheter. Isthmus block was confirmed by both the presence of double potentials, and by differential pacing, i.e. pacing on either side of the line of the block. The procedural times reduced progressively, showing a steep learning curve: the first procedure lasted 120 min, the second procedure 90 min, and the third procedure 50 min skin to skin. No complications occurred, and no early recurrence of AFL was registered.
Conclusions
With the routine use of EP ablation procedures for treating tachyarrhythmias, novel technologies have been developed to improve precision and better success rates. CMR-guided CTI ablation has shown to be safe and effective in prospective studies,5,9 and our experience supports these results. More scientific effort is needed to move beyond atrial flutter CTI ablation. Indeed, AF and VT ablation procedures still show a relevant failure rate and could benefit the most from an iCMR approach, especially in terms of substrate identification and RF lesion evaluation. Eventually, robust data on iCMR results would encourage more centres to overcome the initial obstacles encountered in setting up an iCMR centre, possibly leading to better long-term outcomes.
Contributor Information
Giulia De Zan, Department of Translational Medicine, University of Eastern Piedmont, Maggiore della Carità Hospital, Corso Mazzini 18, Novara 28100, Italy; Cardiology Division, Department of Cardiology, Haga Teaching Hospital, Els Borst-Eilersplein 275, Postbus 40551, The Hague 2504 LN, The Netherlands.
Leonardo Calò, Department of Cardiology, Policlinico Casilino, Via Casilina 1049, Rome 00169, Italy.
Alessio Borrelli, Department of Cardiology, Policlinico Casilino, Via Casilina 1049, Rome 00169, Italy.
Marco Guglielmo, Cardiology Division, Department of Cardiology, Haga Teaching Hospital, Els Borst-Eilersplein 275, Postbus 40551, The Hague 2504 LN, The Netherlands; Department of Cardiology, Division of Heart and Lungs, Utrecht University, Utrecht University Medical Center, Heidelberglaan 100, Utrecht 3584 CX, The Netherlands.
Ermenegildo De Ruvo, Department of Cardiology, Policlinico Casilino, Via Casilina 1049, Rome 00169, Italy.
Sophie Rier, Cardiology Division, Department of Cardiology, Haga Teaching Hospital, Els Borst-Eilersplein 275, Postbus 40551, The Hague 2504 LN, The Netherlands.
Vincent van Driel, Cardiology Division, Department of Cardiology, Haga Teaching Hospital, Els Borst-Eilersplein 275, Postbus 40551, The Hague 2504 LN, The Netherlands.
Hemanth Ramanna, Cardiology Division, Department of Cardiology, Haga Teaching Hospital, Els Borst-Eilersplein 275, Postbus 40551, The Hague 2504 LN, The Netherlands; Department of Medical Technology, The Hague University of Applied Sciences, Johanna Westerdijkplein 75, The Hague 2521 EN, The Netherlands.
Giuseppe Patti, Department of Translational Medicine, University of Eastern Piedmont, Maggiore della Carità Hospital, Corso Mazzini 18, Novara 28100, Italy.
Marco Rebecchi, Department of Cardiology, Policlinico Casilino, Via Casilina 1049, Rome 00169, Italy.
Armando Fusco, Department of Radiology, Policlinico Casilino, Via Casilina 1049, Rome 00169, Italy.
Matteo Stefanini, Department of Radiology, Policlinico Casilino, Via Casilina 1049, Rome 00169, Italy.
Giovanni Simonetti, Department of Radiology, Policlinico Casilino, Via Casilina 1049, Rome 00169, Italy.
Ivo van der Bilt, Cardiology Division, Department of Cardiology, Haga Teaching Hospital, Els Borst-Eilersplein 275, Postbus 40551, The Hague 2504 LN, The Netherlands; Department of Cardiology, Division of Heart and Lungs, Utrecht University, Utrecht University Medical Center, Heidelberglaan 100, Utrecht 3584 CX, The Netherlands.
Funding
None declared.
Data availability
No new data were generated or analysed in support of this research.
References
- 1. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C. et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European association for cardio-thoracic surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European society of cardiology (ESC) developed with the special contribution of the European heart rhythm association (EHRA) of the ESC. Eur Heart J 2021;42:373–498. [DOI] [PubMed] [Google Scholar]
- 2. Erhard N, Metzner A, Fink T. Late arrhythmia recurrence after atrial fibrillation ablation: incidence, mechanisms and clinical implications. Herzschrittmacherther Elektrophysiol 2022;33:71–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rier SC, Vreemann S, Nijhof WH, van Driel V, van der Bilt IAC. Interventional cardiac magnetic resonance imaging: current applications, technology readiness level, and future perspectives. Ther Adv Cardiovasc Dis 2022;16:17539447221119624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bauer BK, Meier C, Bietenbeck M, Lange PS, Eckardt L, Yilmaz A. Cardiovascular magnetic resonance-guided radiofrequency ablation: where are we now? JACC Clin Electrophysiol 2022;8:261–274. [DOI] [PubMed] [Google Scholar]
- 5. Paetsch I, Sommer P, Jahnke C, Hilbert S, Loebe S, Schoene K et al.. Clinical workflow and applicability of electrophysiological cardiovascular magnetic resonance-guided radiofrequency ablation of isthmus-dependent atrial flutter. Eur Heart J Cardiovasc Imaging 2019;20:147–156. [DOI] [PubMed] [Google Scholar]
- 6. McGann C, Kholmovski E, Blauer J, Vijayakumar S, Haslam T, Cates J et al. . Dark regions of no-reflow on late gadolinium enhancement magnetic resonance imaging result in scar formation after atrial fibrillation ablation. J Am Coll Cardiol 2011;58:177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Krahn PRP, Singh SM, Ramanan V, Biswas L, Yak N, Anderson KJT et al. . Cardiovascular magnetic resonance guided ablation and intra-procedural visualization of evolving radiofrequency lesions in the left ventricle. J Cardiovasc Magn Reson 2018;20:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guttman MA, Tao S, Fink S, Kolandaivelu A, Halperin HR, Herzka DA.. Non-contrast-enhanced T(1) -weighted MRI of myocardial radiofrequency ablation lesions. Magn Reson Med 2018;79:879–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ulbrich S, Huo Y, Tomala J, Wagner M, Richter U, Pu L et al. Magnetic resonance imaging-guided conventional catheter ablation of isthmus-dependent atrial flutter using active catheter imaging. Heart Rhythm O2 2022;3:553–559. doi:10.1016/j.hroo.2022.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analysed in support of this research.