Abstract
Background:
Adult survivors of childhood cancer have poor adherence to nutrition guidelines and inadequate intake of dietary vitamins D and E, potassium, fiber, magnesium, and calcium. The contribution of vitamin and mineral supplement use to total nutrient intake in this population is unclear.
Methods:
We examined the prevalence and dose of nutrient intake among 2,570 adult survivors of childhood cancer participating in the St. Jude Lifetime Cohort Study, and the association of dietary supplement use with treatment exposures, symptom burden, and quality of life.
Results:
Nearly 40% of the adult survivors of cancer survivors reported regular use of dietary supplements. While cancer survivors who used dietary supplements were less likely to have inadequate intake of several nutrients, they were also more likely to have excessive intake (total nutrient intake ≥Tolerable Upper Intake Levels) of folate (15.4% vs. 1.3%), vitamin A (12.2% vs. 0.2%), iron (27.8% vs. 1.2%), zinc (18.6% vs. 1%), and calcium (5.1% vs. 0.9%) compared to survivors who did not use dietary supplements (all P values<0.05). Treatment exposures, symptom burden, and physical functioning were not associated with supplement use whereas emotional well-being and vitality were positively associated with supplement use among childhood cancer survivors.
Conclusions:
Supplement use is associated with both inadequate and excessive intake of specific nutrients, but positively impacts aspects of quality of life among childhood cancer survivors.
Keywords: Dietary supplements, Childhood Cancer Survivors, Nutrients, Inadequate Intake, Excess Intake
Condensed Abstract:
Adult survivors of childhood cancer who used dietary supplements were less likely to have inadequate intake of several nutrients but were also more likely to have excessive intake of specific nutrients compared to those who did not use dietary supplements. Healthcare providers need to monitor dietary supplement use among long-term survivors of childhood cancer.
INTRODUCTION
Poor adherence to nutrition recommendations is reported among adult survivors of childhood cancer, including inadequate dietary intake of vitamins D and E, potassium, fiber, magnesium, and calcium.1 Dietary supplement use may help alleviate the nutrition deficiency that cancer survivors experience as a result of cancer treatment or changes in intake patterns during and after treatment. However, studies have indicated potential interactions between dietary supplements and cancer treatment, some of which may lead to long-term negative health consequences2. Cancer survivors have been recommended to obtain adequate nutrients from balanced diets but not dietary supplements.3 Despite this recommendation, use of new supplements post-diagnosis has been reported among 15–50% of the cancer patients.4, 5 Therefore, evaluation of supplement use among adult survivors of childhood cancer is important to determine the contribution of supplement use to total nutrient intake.
The purpose of this study was to investigate the prevalence of dietary supplement use and combined nutrient intake from supplements and nutrition in a large sample of adult survivors of childhood cancer enrolled in the St. Jude Lifetime Cohort (SJLIFE) study. We evaluated whether supplement use was associated with lower or higher prevalence of inadequate and/or excessive nutrient intake among adult survivors of childhood cancer, and the association of dietary supplement use with patient characteristics, treatment exposure, symptom burden, and health-related quality of life (HRQOL).
METHODS
Study Population
The St. Jude Lifetime Cohort (SJLIFE) is a retrospective cohort study of childhood cancer survivors with prospective follow-up and ongoing accrual that aims to systematically and prospectively assess health outcomes among childhood cancer survivors as they age. The design and methodology for this study have been previously described.6, 7 This analysis focused on childhood cancer survivors treated at St. Jude who were 18+ years of age and 10+ years from diagnosis. Among 4,421 eligible survivors, 2,600 completed comprehensive health questionnaires and clinical assessments at SJLIFE. Dietary records with total caloric intake exceeding three standard deviations, above or below the mean natural log-transformed caloric intake in our study population (n=30), were defined as unreliable dietary reporting and excluded, resulting in 2,570 survivors for analysis. Community controls were recruited from the same general geographic area as the survivors and matched to survivors on 5-year age blocks by sex; a total of 357 community controls with reliable reporting for dietary intake were included in this analysis. All participants provided informed consent to participate in this Institutional Review Board-approved study.
Dietary Supplement Use and Nutrient Intake from Supplements
Dietary supplement use was assessed using a self-administered Block 2005 Food Frequency questionnaire (FFQ). Participants were asked about the frequency and duration of three multivitamin/mineral and 10 individual nutrient supplements that they took on a regular basis. Frequency was asked in five categories (didn’t take, a few days per month, 1–3 days per week, 4–6 days per week, and every day) and duration was asked in six categories (less than 1 year, 1 year, 2 years, 3–4 years, 5–9 years, and 10+ years). Participants who reported not taking supplements were defined as non-users. Levels of supplemental nutrient intake were estimated by combining the frequency, duration, and the common dosage of individual nutrients in the supplements. We summed nutrient intake from multivitamin/mineral and individual nutrient supplements to estimate the total nutrient intake from supplements for each study participants.8 Use of any dietary supplement was defined as the use of either multivitamin/mineral (MVM) supplement or single vitamin or mineral supplement, not including prenatal vitamin use. We defined MVM supplement use as taking three or more vitamins with or without minerals from supplements.9, 10
Nutrient Intake from Foods
Dietary intake of study participants was assessed using the 2005 Block FFQ in which the consumption of 110 food items during the past 12 months were asked, which represented their usual or current intake as long-term cancer survivors. For each food item, the FFQ assessed both the frequency and portion size of the consumption. There were eight categories provided as frequency options (never or hardly ever, once a month, 2–3 times a month, once a week, 2–3 times a week, 4–6 times a week, once a day, and 2+times a day). To estimate portion size, pictures were included in the FFQ to facilitate quantification and enhance reporting accuracy. The Block Dietary Data Systems processed the completed FFQs, and estimated nutrient intake using a food list from National Health and Nutrition Examination Survey (NHANES)11 and food composition values for nutrients from the US Department of Agriculture Food and Nutrient Database for Dietary Studies (FNDDS).12 Validation of the Block FFQ was conducted previously by comparing the intakes estimated from the FFQ to the intakes estimated from several 24-hour dietary recalls, and the validation results indicated reasonable ranges (e.g., 0.4 to 0.7) of correlations for most nutrients.13–15
Inadequate and Excess Nutrient Intake
Nutrient intake levels were considered inadequate if the intake levels of total nutrient from both foods and supplements did not meet the amount specified by the Estimated Average Requirements (EAR) or Adequate Intakes (AI) according to the Dietary Reference Intakes (DRIs).16, Nutrient intake levels from both foods and supplements exceeding the Tolerable Upper Intake Levels (UL) defined in the DRIs were considered as excess.
Covariates
Relevant information on cancer diagnosis and treatment exposures were abstracted from medical records of childhood cancer survivors enrolled in the cohort; these included surgical procedures and cumulative doses for 32 specific chemotherapeutic agents as well as radiation treatment fields, dose, and energy source. Demographics and self-reported health behaviors such as alcohol intake, cigarette smoking and physical activity were collected through a series of health questionnaires completed by participants. Using a wall-mounted stadiometer and an electronic scale, we measured and recorded participants’ heights and weights. Moreover, consistent with a previous report, self-reported symptoms were assessed using items from a comprehensive health survey, and symptom presence was denoted if participants endorsed “yes, the condition is still present” for any item measuring that particular symptom.17 HRQOL was measured using the Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36) version 2.18
Levels of physical activity were assessed using minutes per week of moderate-to-vigorous physical activity; vigorous physical activities were weighted at 1.67 per 1 minute.19 Participants who met or exceeded 150 minutes per week of moderate-to-vigorous physical activity were considered as physically active.20 Body mass index (BMI) was calculated by dividing weight in kilograms (kg) by height in meters squared (m2) with adjustments of amputation status. Participants who reported smoking at least 100 cigarettes during their lifetime are considered as smokers, who were further classified as current or former smokers depending on whether they reported on currently smoking. Participants who drank alcohol in the past 12 months were considered as drinkers. Women who had ≤ 1 vs. >1 drink/day and men who had ≤ 2 vs. >2 drinks/day were further classified as moderate vs. high alcohol drinkers.21
Statistical Analysis
We compared the prevalence of multivitamin/mineral (MVM) supplement use, any supplement use, and the individual supplement use of 11 vitamins, 6 minerals, and 2 other supplements between childhood cancer survivors and the community controls (all adults) using the Chi-square test. The mean levels of nutrient intake from foods and from supplements were compared between cancer survivors and community controls using the analysis of variance. We further compared the prevalence of inadequate and excess nutrient intake among the survivors stratified by supplement use. For all abovementioned comparisons, we applied the Benjamin-Hochberg method to control false discovery rate and adjust p values for multiple comparisons22, 23.
Associations between dietary supplement use among adult survivors of childhood cancer with demographic, cancer- and treatment-related characteristics, lifestyle factors, count of symptoms, HRQOL was examined using analysis of variance for continuous variables and Chi-square test for categorical variables. We used the Healthy Eating Index (HEI)-2015, which measures how well Americans adhere to the 2015 Dietary Guidelines, to estimate diet quality.24 The physical component summary scale (PCS) and mental component summary scale (MCS) of HRQOL were standardized (mean=50 and a SD=10), adjusting for age and sex. For factors associated with dietary supplement, we performed multivariable logistic regression models and estimated odds ratios (ORs) and 95% confidence intervals (CIs). To reduce confounding bias and over-adjustment, we applied directed acyclic graphs in which a minimally sufficient set of covariates for each variable was identified. All tests were two sided and p values less than 0.05 was considered statistically significant. SAS version 9.4 (SAS Institute Inc. Cary, NC, USA) were used for all analysis.
RESULTS
Compared to the community controls, the 2,570 adult survivors of childhood cancer were younger and more likely to be males. Leukemia was the most common diagnosis among the survivors, followed by lymphoma, embryonal tumors, sarcoma, CNS tumors, and others (eTable 1).
The prevalence of any individual supplement use and MVM supplement use was 38.3% (95% CI: 36.4% - 40.2%) and 30.2% (95% CI: 28.4%−31.9%), respectively, among adult survivors of childhood cancer, which was not significantly different from that in the community controls (any supplement use: 45.5%, 95% CI: 40.2%−50.6%, P=0.06; MVM supplement use: and 37.3%, 95% CI: 32.3–42.3%, P=0.05), after adjustment of age, gender and race/ethnicity (Table 1). Among those who reported regular use of any dietary supplements (i.e., users), adult cancer survivors reported higher doses than community controls for vitamin C (323.3 vs. 244.9 mg/d, P=0.003) and calcium (494.1 vs. 377.0 mg/d, P=0.002) from dietary supplements. Overall, adult survivors of childhood cancer had higher levels of dietary intake of vitamin D (165.4 vs. 141.1 IU/d, P=0.003), calcium (906.3 vs. 806.2 mg/d, P=0.003), and omega-3 fatty acid (1.7 vs. 1.5 g/d, P=0.02) than community controls.
Table 1.
Levels of Nutrient Intake from Foods and Supplements among Adult survivors of Childhood Cancer and Community Controls
| Nutrients1 | Prevalence of Supplement Use (n=2927) (%, 95% CI) | P value1 | Nutrient Intake from Foods (n=2927) (Mean ± SE) | P Value1 | Nutrient Intake from Supplements Among Users (n=1146) (Mean ± SE) | P value1 | |||
|---|---|---|---|---|---|---|---|---|---|
| Childhood Cancer Survivors (n=2,570) | Community Controls (n=357) | Childhood Cancer Survivors (n=2,570) | Community Controls (n=357) | Childhood Cancer Survivors (n=984) | Community Controls (n=162) | ||||
| Any supplement use 2 | 38.3 (36.4–40.2) | 45.4 (40.2–50.6) | 0.06 | -- | -- | -- | -- | -- | -- |
| MVM supplement use 3 | 30.2 (28.4–31.9) | 37.3 (32.2–42.3) | 0.05 | -- | -- | -- | -- | -- | -- |
| Supplement use of individual nutrients 4 | |||||||||
| Vitamin A (RAE, μg/d) | 30.7 (28.9–32.4) | 35.9 (30.9–40.9) | 0.21 | 823.3 ± 10.5 | 779.6 ± 21.3 | 0.24 | 1576.7 ± 39.3 | 1513.4 ± 84.6 | 0.78 |
| Beta-carotene (μg/d) | 27.6 (25.9–29.3) | 32.2 (27.3–37.1) | 0.27 | 3912.2 ± 72.4 | 4327.2 ± 186.1 | 0.12 | 2562.6 ± 215.4 | 1625.1 ± 382.4 | 0.19 |
| Thiamin (mg/d) | 32.0 (30.2–33.8) | 39.5 (34.4–44.6) | 0.11 | 1.6 ± 0.02 | 1.5 ± 0.04 | 0.07 | 3.2 ± 0.1 | 4.0 ± 0.4 | 0.65 |
| Riboflavin (mg/d) | 32.0 (30.2–33.8) | 39.5 (34.4–44.6) | 0.11 | 2.2 ± 0.02 | 2.0 ±0.05 | 0.11 | 3.3 ± 0.1 | 4.2 ± 0.4 | 0.65 |
| Niacin (mg/d) | 32.0 (30.2–33.8) | 39.5 (34.4–44.6) | 0.11 | 23.4 ± 0.3 | 21.2 ± 0.6 | 0.10 | 35.8 ± 1.3 | 44.3 ± 3.7 | 0.65 |
| Vitamin B6 (mg/d) | 32.0 (30.2–33.8) | 39.5 (34.4–44.6) | 0.11 | 2.0 ± 0.02 | 1.9 ± 0.1 | 0.19 | 2.6 ± 0.1 | 3.0 ± 0.2 | 0.78 |
| Folate (DFE, μg/d) | 32.8 (31.0–34.6) | 39.8 (34.7–44.9) | 0.12 | 542.1 ± 6.2 | 519.2 ± 14.3 | 0.53 | 814.2 ± 18.9 | 862.31 ± 51.3 | 0.99 |
| Vitamin B12 (μg/d) | 32.0 (30.2–33.8) | 39.5 (34.4–44.6) | 0.11 | 5.8 ± 0.08 | 5.0 ± 0.2 | 0.05 | 6.7 ± 0.2 | 7.6 ± 0.5 | 0.80 |
| Vitamin C (mg/d) | 35.4 (33.6–37.3) | 42.6 (37.4–47.7) | 0.12 | 108.5 ± 1.6 | 94.8 ± 3.1 | 0.05 | 323.3 ± 14.4 | 244.9 ± 20.2 | 0.003 |
| Vitamin D (IU/d) | 34.1 (32.3–36.0) | 40.9 (35.8–46.0) | 0.12 | 165.4 ± 2.6 | 141.1 ± 6.4 | 0.003 | 334.6 ± 6.2 | 342.3 ± 15.9 | 0.80 |
| Vitamin E (mg/d) | 33.1 (31.3–34.9) | 39.8 (34.7–44.9) | 0.14 | 8.1 ± 0.1 | 8.2 ± 0.2 | 0.42 | 29.9 ± 2.0 | 21.7 ± 1.6 | 0.06 |
| Calcium (mg/d) | 34.1 (32.3–35.9) | 38.4 (33.3–43.4) | 0.38 | 906.3 ± 9.6 | 806.2 ± 20.3 | 0.003 | 494.1 ± 14.1 | 377.0 ± 30.2 | 0.002 |
| Selenium (μg/d) | 29.8 (28.1–31.6) | 35.9 (30.9–40.9) | 0.15 | 108.8 ± 1.3 | 97.8 ±2.7 | 0.08 | 32.8 ± 1.5 | 36.2 ± 4.3 | 0.80 |
| Iron (mg/d) | 30.8 (29.0–32.6) | 35.6 (30.6–40.6) | 0.27 | 14.9 ± 0.2 | 13.4 ± 0.3 | 0.05 | 26.5 ± 0.9 | 26.4 ± 2.2 | 0.80 |
| Zinc (mg/d) | 30.4 (28.6–32.1) | 37.0 (31.9–42.0) | 0.12 | 12.6 ± 0.2 | 11.3 ± 0.3 | 0.10 | 18.7 ± 0.6 | 18.9 ± 1.6 | 0.80 |
| Copper (mg/d) | 29.6 (27.8–31.4) | 35.3 (30.3–40.3) | 0.18 | 1.4 ± 0.01 | 1.4 ± 0.03 | 0.42 | 1.5 ± 0.03 | 1.6 ± 0.1 | 0.80 |
| Magnesium (mg/d) | 27.1 (25.4–28.8) | 32.2 (27.3–37.1) | 0.21 | 308.0 ± 3.2 | 296.2 ± 7.0 | 0.44 | 75.1 ± 1.2 | 75.9 ± 2.7 | 0.80 |
| Omega-3 fatty acids (g/d) | 11.8 (10.5–13.0) | 16.5 (12.7–20.4) | 0.11 | 1.7 ± 0.02 | 1.5 ± 0.04 | 0.02 | 0.4 ± 0.01 | 0.4 ± 0.02 | 0.99 |
| Omega-6 fatty acids (g/d) | 11.8 (10.5–13.0) | 16.5 (12.7–20.4) | 0.11 | 15.6 ± 0.2 | 14.2 ±0.4 | 0.05 | 0.1 ± 0.002 | 0.1 ± 0.004 | 0.99 |
Abbreviations: MVM, multivitamin/mineral
Adjusted for age, sex, and race/ethnicity. All comparisons were adjusted for false discovery rate (FDR). P values < 0.05 were bolded.
Any supplement use was defined as the use of multiple vitamins or single vitamins, not including prenatal vitamin use.
MVM supplement use was defined as containing 3 or more vitamins with or without minerals. B-complex vitamin use was considered as one type of multivitamin use.
Individual supplements included the ingredients as single supplements and as part of MVM.
More than half of the survivors were consuming inadequate level of (total nutrient intake <EAR or AI) vitamin D (74.2%), vitamin E (62.0%), and magnesium (50.5%) (Figure 1). The prevalence of inadequate intake for these nutrients was much lower among survivors who used dietary supplements than those who did not (vitamin D: 34.9 vs. 94.6%; vitamin E: 14.2 vs. 85.6%; magnesium: 27.4 vs. 59.0%) (all P values < 0.0001) (Table 2). For many nutrients (vitamins A, B, and C, selenium, iron, zinc, and copper), the prevalence of inadequate nutrient intake was below 2% among users. However, when nutrients from supplement sources were not accounted (i.e., foods alone), the prevalence of inadequate nutrition intake was still significantly lower among survivors who used dietary supplements than those who did not (all P values<0.05).
Figure 1.
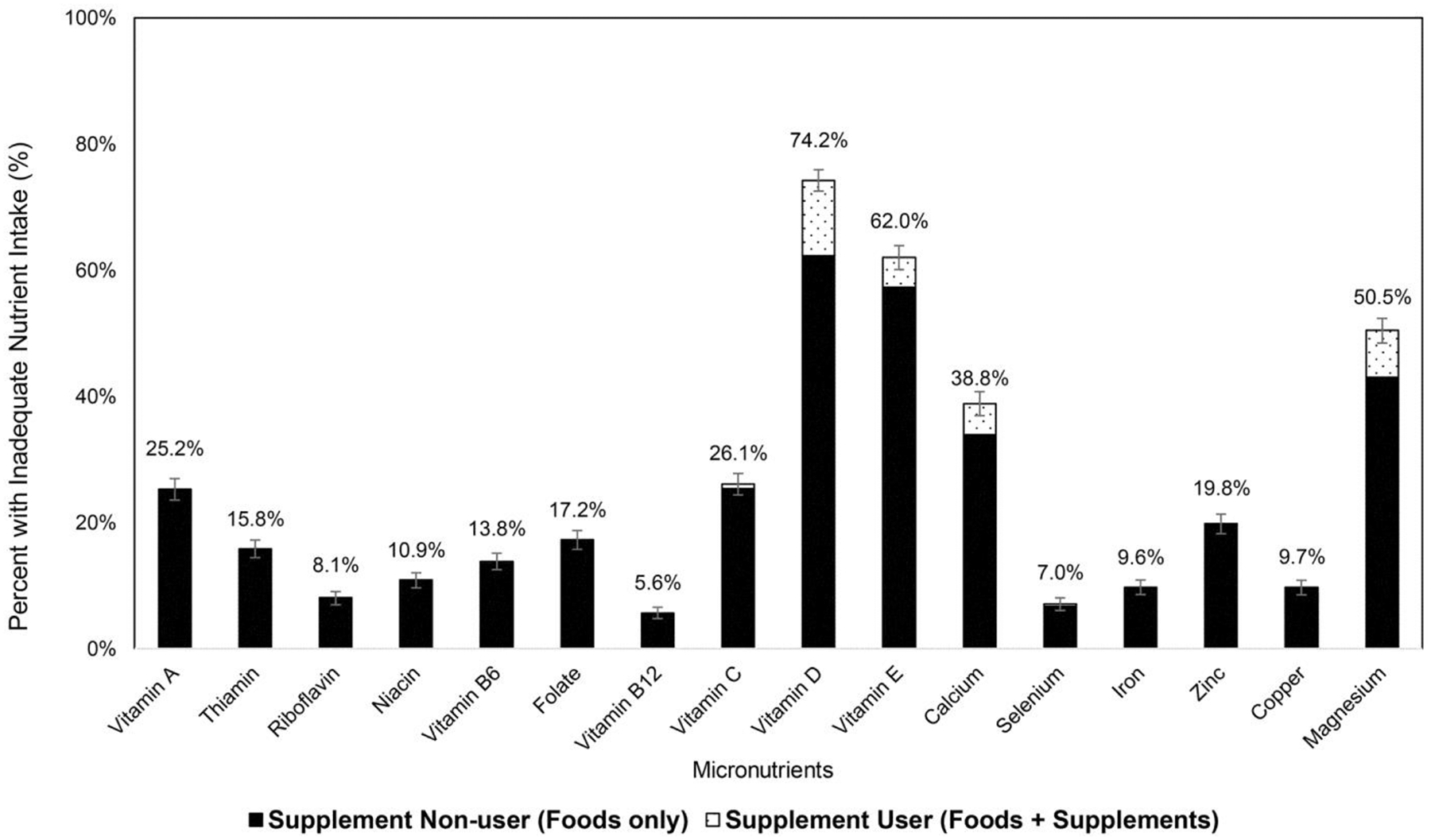
Percent of adult survivors of childhood cancer with inadequate nutrient intake (total intake level < estimated average requirement). Number above the bar represents the percent of inadequate nutrient among all adult survivors of childhood cancer, including both supplement users and nonusers. The bar in black corresponds to the percent of inadequate nutrient intake among supplement nonusers; the bar in white with dotted pattern corresponds to the percent of inadequate nutrient intake among supplement users.
Table 2.
Adults Survivors of Childhood Cancer with Inadequate (Intake Level < Estimated Average Requirements) or Excess Nutrient Intake (Intake > Tolerable Upper Intake) by Source
| Nutrients4 | Percent (%) of Survivors with Inadequate Nutrient Intake | Percent (%) of Survivors with Excess Nutrient Intake | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Non-users | Users | Non-users | Users | |||||||
| Foods Alone1 | Foods Alone2 | Foods + Supplements3 | Foods Alone1 | Foods Alone2 | Foods + Supplements3 | |||||
| n | % (95% CI) | n | % (95% CI) | % (95% CI) | n | % (95% CI) | n | % (95% CI) | % (95% CI) | |
| Vitamin A (RAE, μg) | 1782 | 36.4 (34.1–38.6) | 788 | 25.4 (22.3–28.4) | 0.0 (0.0–0.0) | 1782 | 0.2 (0.005–0.4) | 788 | 0.4 (0.0–0.8) | 12.2 (9.9–14.5) |
| Thiamin (mg) | 1748 | 23.0 (21.0–25.0) | 822 | 14.8 (12.4–17.3) | 0.5 (0.0–1.0) | 1748 | --- | 822 | --- | --- |
| Riboflavin (mg) | 1748 | 11.7 (10.2–13.2) | 822 | 5.1 (3.6–6.6) | 0.2 (0.0–0.6) | 1748 | --- | 822 | --- | --- |
| Niacin (mg) | 1748 | 15.8 (14.1–17.6) | 822 | 9.4 (7.4–11.4) | 0.2 (0.0–0.6) | 1748 | --- | 822 | --- | 26.3 (23.3–29.3) |
| Vitamin B6 (mg) | 1748 | 20.2 (18.3–22.1) | 822 | 12.8 (10.5–15.1) | 0.2 (0.0–0.6) | 1748 | 0.0 (0.0–0.0) | 822 | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) |
| Folate (DFE, μg) | 1727 | 25.5 (23.4–27.5) | 843 | 18.4 (15.8–21.0) | 0.2 (0.0–0.6) | 1727 | 1.3 (0.7–1.8) | 843 | 0.9 (0.3–1.6) | 15.4 (13.0–17.9) |
| Vitamin B12 (μg) | 1748 | 8.3 (7.0–9.6) | 822 | 5.7 (4.1–7.3) | 0.0 (0.0–0.0) | 1748 | --- | 822 | --- | --- |
| Vitamin C (mg) | 1660 | 39.3 (37.0–41.7) | 910 | 28.0 (25.1–30.9) | 1.9 (1.0–2.7) | 1660 | 0.0 (0.0–0.0) | 910 | 0.0 (0.0–0.0) | 1.6 (0.8–2.5) |
| Vitamin D (IU) | 1693 | 94.6 (93.5–95.6) | 877 | 92.9 (91.2–94.6) | 34.9 (31.7–38.1) | 1693 | 0.0 (0.0–0.0) | 877 | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) |
| Vitamin E (mg) | 1720 | 85.6 (83.9–87.2) | 850 | 82.7 (80.2–85.3) | 14.2 (11.9–16.6) | 1720 | --- | 850 | --- | 0.0 (0.0–0.0) |
| Calcium (mg) | 1694 | 51.4 (49.0–53.7) | 876 | 46.9 (43.6–50.2) | 14.5 (12.2–16.8) | 1694 | 0.9 (0.5–1.4) | 876 | 1.3 (0.5–2.0) | 5.1 (3.7–6.6) |
| Selenium (μg) | 1803 | 9.5 (8.1–10.8) | 767 | 5.3 (3.8–6.9) | 1.2 (0.4–1.9) | 1803 | 0.8 (0.4–1.2) | 767 | 0.8 (0.2–1.4) | 1.3 (0.5–2.1) |
| Iron (mg) | 1779 | 13.9 (12.3–15.5) | 791 | 10.0 (7.9–12.1) | 0.1 (0.0–0.4) | 1779 | 1.2 (0.7–1.8) | 791 | 1.1 (0.4–1.9) | 27.8 (24.7–30.9) |
| Zinc (mg) | 1790 | 28.3 (26.2–30.4) | 780 | 23.1 (20.1–26.0) | 0.3 (0.0–0.6) | 1790 | 1.0 (0.5–1.5) | 780 | 1.5 (0.7–2.4) | 18.6 (15.9–21.3) |
| Copper (mg) | 1809 | 13.8 (12.2–15.4) | 761 | 6.4 (4.7–8.2) | 0.0 (0.0–0.0) | 1809 | 0.0 (0.0–0.0) | 761 | 0.1 (0.0–0.4) | 0.1 (0.0–0.4) |
| Magnesium (mg)5 | 1874 | 59.0 (56.8–61.2) | 696 | 51.1 (47.4–54.9) | 27.4 (24.1–30.8) | 1874 | --- | 696 | --- | 0.0 (0.0–0.0) |
Abbreviations: RAE, retinol activity equivalents; DFE, dietary folate equivalents
Foods alone for non-users correspond to nutrient intake obtained from food sources, representing total nutrient intake among non-users.
Foods alone for users correspond to nutrient intake obtained from food sources (foods alone) among users. After adjustments of false discovery rates, the percent of survivors with inadequate nutrient intake based on foods alone was significantly lower among users than non-users (all P-values were < 0.05); the percent of survivors with excess nutrient intake based on foods alone did not differ between users and non-users (all P-values were >0.05).
Foods + supplements for users correspond to total nutrient intake obtained from both food and supplement sources, representing total nutrient intake among users. After adjustments of false discovery rates, the percent of survivors with inadequate nutrient intake based on total nutrient intake was significantly lower among users than non-users; the percent of survivors with excess nutrient intake based on total nutrient intake was significantly higher among users than non-users (all P-values<0.0001) except for selenium (P-value = 0.07) and copper (P-value = 0.1).
UL for vitamin A applies to preformed vitamin A only; ULs for niacin and folate apply to synthetic forms obtained from supplements, fortified foods, or a combination of the two (niacin intake from fortified foods is not available in this analysis); vitamin E in this analysis was assessed as α-tocopherol and UL for vitamin E applies to any form of supplemental α-tocopherol (vitamin E intake from fortified foods is not available in this analysis); UL for magnesium represents intake from a pharmacological agent only and does not include intake from food and water; no UL is available for thiamin, riboflavin, and vitamin B12.
The prevalence of excess nutrient intake (total nutrient intake ≥ UL) was below 5% for most nutrients except for niacin (8.4%), folate (5.1%), iron (9.4%), and zinc (6.3%) (Figure 2). However, the prevalence of excess nutrient intake was all below 2% among survivors who did not use dietary supplements (non-users) or when nutrients from food source alone (foods alone among users) were considered (Table 2). When nutrient intake from dietary supplements was also considered, the prevalence of excessive nutrient intake was above 5% for vitamin A, folate, iron, zinc, and calcium, which was significantly higher among users than non-users (vitamin A: 12.2 vs. 0.2%; folate:15.4 vs. 1.3%; iron: 27.8 vs. 1.2%; zinc: 18.6 vs. 1.0%; calcium: 5.1 vs. 0.9%) (all P-values <0.0001).
Figure 2.
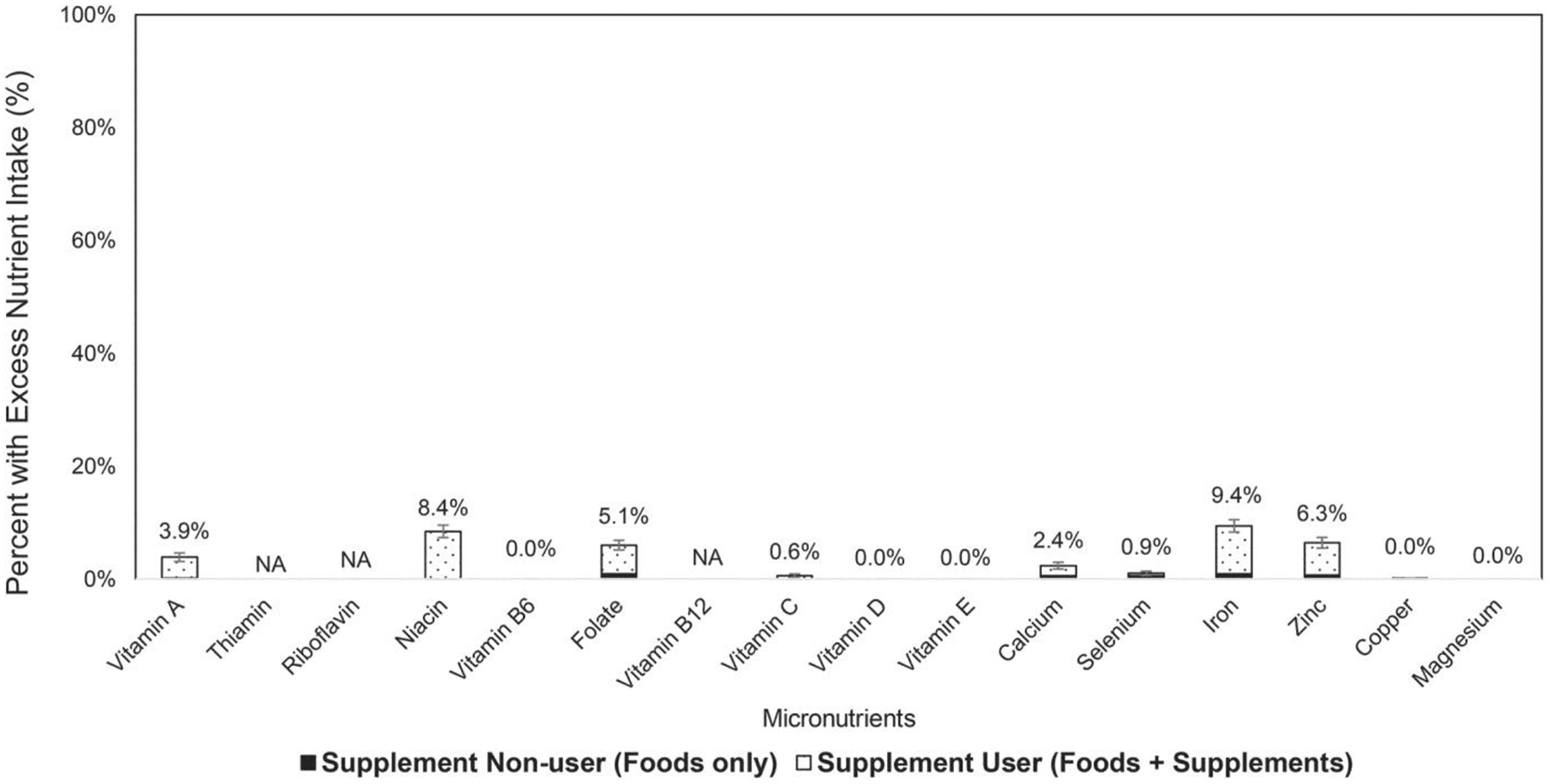
Percent of adult survivors of childhood cancer with excess nutrient intake (total intake level > tolerable upper intake level). Number above the bar represents the percent of excess nutrient among all adult survivors of childhood cancer, including both supplement users and nonusers. The bar in black corresponds to the percent of excess nutrient intake among supplement nonusers; the bar in white with dotted pattern corresponds to the percent of excess nutrient intake among supplement users. UL for vitamin A applies to preformed vitamin A only; UL for niacin and folate applies to synthetic forms obtained from supplements, fortified foods, or a combination of the two (niacin intake from fortified foods is not available in this analysis); vitamin E is assessed as α-tocopherol; and UL for vitamin E applies to any form of supplemental α-tocopherol (vitamin E intake from fortified foods is not available in this analysis); UL for magnesium represents intake from a pharmacological agent only and does not include intake from food and water; no UL is available for thiamin, riboflavin, and vitamin B12. UL, tolerable upper intake level.
Compared to childhood cancer survivors who were non-users, dietary supplements were older and had higher proportions of women, non-Hispanic Whites, people with more advanced education degrees and higher income; in addition, they were more likely to have insurance coverage and utilize general healthcare (Table 3). They were also less likely to be smokers or obese, and more likely to be physically active, use practice sun protection behaviors and have better diet quality. Supplement users and nonusers did not differ by primary cancer diagnosis and treatment exposure, although survivors who were more than 30 years from diagnosis were less likely to use dietary supplements than those who were between 10 and 20 years from diagnosis. Supplement use was not associated with symptom count or the physical domain of the HRQOL. However, cancer survivors who used dietary supplement had a significantly higher mental component summary score (49.0 vs. 47.8, P=0.01) and higher scores in emotional well-being (48.9 vs. 47.7, P=0.01) and vitality (49.2 vs. 48.2, P=0.02) compared to non-users. Similar associations were found comparing cancer survivors who used and did not use MVM supplements (eTable 2).
Table 3.
Factors Associated with Dietary Supplement Use Among Adult Survivors of Childhood Cancer
| Characteristics | Users of Any Supplement (N=984) | Nonuser of Any Supplement (N=1,586) | P value | Odds Ratio (95% CI) Model 11 | Odds Ratio (95% CI) Model 21 |
|---|---|---|---|---|---|
| Age, years, mean (SD) | 33.2 (8.2) | 31.8 (8.3) | <0.0001 | 1.02 (1.01–1.03) | 1.02 (1.01–1.03) |
| Gender, N (%) | |||||
| Men | 483 (49.1) | 884 (53.2) | 0.04 | 1 (Ref) | 1 (Ref) |
| Women | 501 (50.9) | 742 (46.8) | 1.18 (1.01–1.38) | 1.18 (1.01–1.38) | |
| Race/ethnicity, N (%) | |||||
| Non-Hispanic Whites | 850 (86.4) | 1291 (81.4) | 0.001 | 1 (Ref) | 1 (Ref) |
| Other | 134 (13.6) | 295 (18.6) | 0.69 (0.55–0.86) | 0.69 (0.55–0.86) | |
| Education, N (%) | |||||
| Grades 0–12 | 186 (19.9) | 506 (34.1) | <0.0001 | 1 (Ref) | 1 (Ref) |
| Some post high school | 297 (31.8) | 508 (34.2) | 1.59 (1.28–1.98) | 1.62 (1.30–2.03) | |
| College graduate | 451 (48.3) | 472 (31.8) | 2.60 (2.10–3.21) | 2.49 (2.01–3.09) | |
| Family income to poverty ratio (FIPR),2 N (%) | |||||
| Low (FIPR≤1.3) | 171 (20.1) | 385 (29.5) | <0.0001 | 1 (Ref) | 1 (Ref) |
| Median (1.3<FIPR≤3.5) | 318 (37.5) | 590 (45.3) | 1.21 (0.97–1.52) | 1.18 (0.94–1.49) | |
| High (FIPR>3.5) | 360 (42.4) | 329 (25.2) | 2.46 (1.95–3.11) | 2.35 (1.85–2.98) | |
| Insurance coverage, N (%) | |||||
| Insured | 802 (83.5) | 1127 (73.7) | <0.0001 | 1 (Ref) | 1 (Ref) |
| Uninsured | 158 (16.5) | 401 (26.2) | 0.55 (0.45–0.68) | 0.59 (0.48–0.73) | |
| General healthcare utilization,3 N (%) | |||||
| Yes | 896 (91.1) | 1311 (82.7) | <0.0001 | 1 (Ref) | 1 (Ref) |
| No | 88 (8.9) | 275 (17.3) | 0.47 (0.36–0.60) | 0.51 (0.40–0.67) | |
| Current marital status,4 N (%) | |||||
| Unmarried | 483 (49.1%) | 851 (53.7%) | 0.02 | 1 (Ref) | 1 (Ref) |
| Married | 501 (50.9%) | 735 (46.3%) | 1.20 (1.02–1.41) | 1.05 (0.88–1.24) | |
| Smoking,5 N (%) | |||||
| Nonsmokers | 673 (68.9) | 992 (63.4) | <0.0001 | 1 (Ref) | 1 (Ref) |
| Former smokers | 133 (13.6) | 159 (10.2) | 1.23 (0.96–1.58) | 1.13 (0.86–1.48) | |
| Current smokers | 171 (17.5) | 414 (26.5) | 0.61 (0.50–0.75) | 0.69 (0.55–0.87) | |
| Alcohol, drink/week, N (%) | |||||
| Nondrinkers | 452 (46.7) | 736 (47.8) | 0.23 | 1 (Ref) | 1 (Ref) |
| Moderate drinkers | 389 (40.2) | 639 (41.5) | 0.99 (0.84–1.18) | 0.95 (0.79–1.15) | |
| Heavy drinkers | 126 (13.0) | 166 (10.8) | 1.24 (0.95–1.60) | 1.15 (0.86–1.53) | |
| Diet quality (Health Eating Index −2015),6 N (%) | |||||
| Q1 (HEI<52.2) | 146 (14.8) | 497 (31.3) | <0.0001 | 1 (Ref) | 1 (Ref) |
| Q2 (52.2≤HEI<59.7) | 212 (21.5) | 430 (27.1) | 1.68 (1.31–2.15) | 1.68 (1.28–2.20) | |
| Q3 (59.7≤HEI<67.9) | 269 (27.3) | 373 (23.5) | 2.46 (1.93–3.13) | 2.23 (1.70–2.92) | |
| Q4 (HEI≥67.9) | 357 (36.3) | 286 (18.0) | 4.25 (3.34–5.41) | 3.49 (2.64–4.61) | |
| Physical activity,7 N (%) | |||||
| Active | 520 (53.4) | 750 (48.4) | 0.01 | 1 (Ref) | 1 (Ref) |
| Inactive | 453 (46.6) | 800 (51.6) | 0.82 (0.70–0.96) | 0.75 (0.63–0.90) | |
| Sun exposure protection,8 N (%) | |||||
| Yes | 767 (78.8) | 1116 (71.9) | 0.0001 | 1 (Ref) | 1 (Ref) |
| No | 207 (21.3) | 437 (28.1) | 0.69 (0.57–0.83) | 0.75 (0.61–0.93) | |
| Body mass index (BMI),9 kg/m2, mean (SD) | 28.2 (7.4) | 28.6 (7.4) | 0.17 | 0.99 (0.98–1.00) | 0.99 (0.98–1.00) |
| Weight status4, N (%) | |||||
| BMI≤24.9 | 384 (39.0) | 560 (35.3) | 0.12 | 1 (Ref) | 1 (Ref) |
| BMI=25–29.9 | 277 (28.2) | 453 (28.6) | 0.89 (0.73–1.09) | 0.87 (0.70–1.09) | |
| BMI≥30 | 323 (32.8) | 573 (36.1) | 0.82 (0.68–0.99) | 0.74 (0.60–0.92) | |
| Primary diagnosis, N (%) | |||||
| Leukemia | 380 (38.7) | 606 (38.2) | 0.64 | 1 (Ref) | 1 (Ref) |
| Lymphoma | 204 (20.8) | 293 (18.5) | 1.11 (0.89–1.38) | 1.04 (0.83–1.31) | |
| Embryonal tumors | 121 (12.3) | 214 (13.5) | 0.90 (0.70–1.17) | 0.93 (0.72–1.20) | |
| Sarcoma | 120 (12.2) | 208 (13.1) | 0.92 (0.71–1.19) | 0.91 (0.70–1.19) | |
| Central nervous system tumors | 92 (9.4) | 147 (9.3) | 1.00 (0.75–1.33) | 1.14 (0.85–1.54) | |
| Other | 64 (6.5) | 117 (7.4) | 0.87 (0.63–1.21) | 0.90 (0.64–1.25) | |
| Time from diagnosis, years, mean (SD) | 24.5 (8.2) | 23.8 (8.0) | 0.05 | 1.01 (1.00–1.02) | 0.99 (0.97–1.00) |
| Time from diagnosis, N (%) | |||||
| 10–19.99 | 322 (32.7) | 584 (36.8) | 0.10 | 1 (Ref) | 1 (Ref) |
| 20–29.99 | 415 (42.2) | 623 (39.3) | 1.21 (1.01–1.45) | 0.99 (0.81–1.21) | |
| ≥30 | 247 (25.1) | 379 (23.9) | 1.18 (0.96–1.46) | 0.72 (0.53–0.98) | |
| Treatment exposures, N (%) | |||||
| Radiation therapy | |||||
| Yes | 602 (61.2) | 930 (58.6) | 0.20 | 1.11 (0.95–1.31) | 1.01 (0.85–1.20) |
| No | 382 (38.8) | 656 (41.4) | 1 (Ref) | 1 (Ref) | |
| Alkylating agents | |||||
| Yes | 648 (65.9) | 979 (61.7) | 0.03 | 1.20 (1.01–1.41) | 1.17 (0.99–1.39) |
| No | 336 (34.2) | 607 (38.3) | 1 (Ref) | 1 (Ref) | |
| Anthracyclines | |||||
| Yes | 571 (58.0) | 928 (58.5) | 0.80 | 0.98 (0.83–1.15) | 1.04 (0.87–1.25) |
| No | 413 (42.0) | 658 (41.5) | 1 (Ref) | 1 (Ref) | |
| Antimetabolites | |||||
| Yes | 521 (53.0) | 848 (53.5) | 0.80 | 0.98 (0.84–1.15) | 0.91 (0.73–1.14) |
| No | 463 (47.1) | 738 (46.5) | 1 (Ref) | 1 (Ref) | |
| Glucocorticoids | |||||
| Yes | 486 (49.4) | 774 (48.8) | 0.77 | 1.02 (0.83–1.20) | 0.95 (0.75–1.20) |
| No | 498 (50.6) | 812 (51.2) | 1 (Ref) | 1 (Ref) | |
| Symptom count,10 N (%) | |||||
| 0–2 | 289 (29.4) | 434 (27.4) | 0.46 | 1 (Ref) | 1 (Ref) |
| 3–6 | 485 (49.3) | 789 (49.8) | 0.92 (0.77–1.11) | 0.87 (0.71–1.08) | |
| 7–11 | 210 (21.3) | 363 (22.9) | 0.87 (0.69–1.09) | 0.87 (0.67, 1.13) | |
| Health-related quality of life (HRQOL),11 mean (SD) | |||||
| Physical Component Summary (PCS) Score | 49.3 (10.4) | 49.2 (10.1) | 0.82 | 1.01 (0.97–1.05) | 0.99 (0.94–1.03) |
| Physical functioning | 48.5 (10.5) | 48.2 (10.6) | 0.47 | 1.01 (0.98–1.05) | 1.00 (0.95–1.04) |
| Role limitation from physical health problems | 50.4 (10.6) | 50.2 (10.6) | 0.68 | 1.01 (0.97–1.05) | 0.99 (0.94–1.03) |
| Pain | 50.7 (10.7) | 50.0 (10.9) | 0.11 | 1.03 (0.99–1.07) | 1.02 (0.98–1.06) |
| Perceived general health | 46.8 (11.8) | 46.4 (11.6) | 0.38 | 1.02 (0.98–1.05) | 1.01 (0.97–1.05) |
| Mental Component Summary (MCS) Score | 49.0 (10.6) | 47.8 (12.1) | 0.01 | 1.05 (1.01–1.09) | 1.04 (1.00–1.08) |
| Emotional well-being | 48.9 (10.4) | 47.7 (11.7) | 0.01 | 1.05 (1.01–1.09) | 1.04 (1.00–1.08) |
| Role limitation from emotional problems | 49.5 (10.6) | 48.5 (11.9) | 0.04 | 1.04 (1.00–1.08) | 1.03 (0.99–1.07) |
| Social functioning | 48.9 (10.3) | 48.1 (11.1) | 0.10 | 1.03 (0.99–1.07) | 1.01 (0.97–1.06) |
| Vitality | 49.2 (10.6) | 48.2 (11.2) | 0.02 | 1.05 (1.01–1.08) | 1.05 (1.01–1.09) |
Model 1 was univariate model of each predictor; Model 2 was generated by using directed acyclic graphs (DAGs). Models for age, gender and race/ethnicity are univariate; models for education, family income, insurance coverage, general healthcare utilization, current marital status, primary diagnosis, and time from diagnosis were adjusted for age, gender, and race/ethnicity as covariates; models for treatment exposure will additionally adjust for cancer type as covariates. Models for behavioral risk factors (smoking, alcohol consumption, diet quality, physical activity, sun exposure protection, and body mass index), symptoms, and HRQOL were adjusted for age, gender, race/ethnicity, education, and treatment exposure as covariates.
Family income to poverty ratio (FIPR) was defined based on household income in relation to the federal poverty threshold.
General health care utilization was defined as “yes” if the participants reported receiving any health care from doctor, hospital, emergency room, other clinic, or other.
Current marital status was defined as “married” if participants answered “Married” or “Living with a partner as married” and as “unmarried” if answered “Single, never married or never lived with parents as married”, “Widowed”, “Divorced”, or “Separated or no longer living as married”.
Smokers will be defined as individuals who reported smoking at least 100 cigarettes during their lifetime, with former smokers defined as not currently smoking and current smokers defined as currently smoking.
Diet quality will be assessed using the Healthy Eating Index-2015 (HEI-2015) and categorized into quartiles based on the quartile distribution.
Minutes per week of moderate-to-vigorous physical activity (MVPA) will be calculated by summarizing minutes, with vigorous physical activities weighted at 1.67 minutes for each minute of vigorous physical activity. Participants will be classified as physically active if MVPA met or exceeded 150 minutes per week, according to the Center for Disease Control and Prevention (CDC) Physical Activity Guidelines for Americans.34
Sun exposure protection was defined based on the questions about “SPF protection”, “wearing protective clothing”, “wearing a hat”, and “staying in the shade”. If answer to any of the questions was “often” or “always”, they were categorized as having sun exposure protection. Note those who answered “never”, “rarely” or “sometimes” were not considered as having sun exposure protection behavior.
Body mass index (BMI) will be calculated by dividing weight in kilograms (kg) by height in meters squared (m2), adjusted for amputation status. Participants will be classified as underweight or normal weight (BMI ≤24.9 kg/m2), overweight (BMI=25–29.9 kg/m2), and obese (BMI ≥30 kg/m2).
Symptoms was created to assess risk-based toxicities as outlined in the Children’s Oncology Group guidelines with demonstrated sensitivity to treatment exposures. Eleven physical symptoms were constructed using items assessed in the Comprehensive Health Survey, and symptom presence was denoted if participants endorsed “yes, the condition is still present” for any item measuring that particular symptom. A summated item score of a particular symptom, when the converted T-score is ≥ 63, will be used to denote symptom presence.17 The presence of eleven symptoms (yes=1 and no=0) were added up and a “symptom count” was generated for each survivor.
Health-related quality of life (HRQOL) was evaluated using SF-36 physical component and mental component summary score.35 The ORs were generated based on every 5-unite change in score.
DISCUSSION
In a large cohort of adult survivors of childhood cancer, we found that nearly 40% of the adult survivors of cancer survivors reported regular use of dietary supplements and endorsed higher doses of vitamin C and calcium from supplements than community controls. While supplement use among cancer survivors were associated with lower prevalence of inadequate total nutrient intake for some nutrients, they also reported excess nutrient intake for other nutrients. Treatment exposures, symptom burden, and physical functioning were not associated with supplement use whereas emotional well-being and vitality were positively associated with supplement use among childhood cancer survivors.
To our knowledge, this study is among the first to assess dietary supplement use in a large cohort of adult survivors of childhood cancer survivors. The reported prevalence of supplement use appeared to be lower than that in previous cohorts of prostate, breast, and colorectal cancer survivors, which ranged between 50% and 85%.4, 9, 25, 26 However, the prevalence of supplement use in adult survivors of childhood was not different from that in community controls who responded to the same questionnaire. Despite no difference in the prevalence of supplement use, survivors took a higher dose of supplements for vitamin C and calcium than the controls. Survivors also had higher intake levels of calcium (but not vitamin C) from foods than the controls. The impact of high calcium intake from supplements and foods among childhood cancer survivors warrant further investigations.
Despite the lower percentage of inadequate nutrient intake attributable to dietary supplement use among childhood cancer survivors, it is important to note that more than half of the adult childhood cancer survivors still had inadequate nutrient intake for several nutrients such as vitamins D and E, and magnesium, and more than one-third (39%) had inadequate intake of calcium. These nutrients are known as “shortfall nutrients”, but very few studies have investigated their impacts on health outcomes and possible consequences among cancer survivors. Nonetheless, existing data have suggested that low vitamin D status adversely affects cancer survival: results from a meta-analysis including six prospective cohort studies with a total of 6,092 breast cancer survivors found a positive association between low circulating levels of 25-hydroxyvitamin D (25(OH)D) and the risk of breast cancer-specific and all-cause mortality.27 Given that inadequate nutrient intake is prevalent among cancer survivors, future studies are warranted to further evaluate its impact on health and whether nutrient supplementation can improve the health outcomes of survivors with nutrient deficiency.28
Cancer survivors who used supplements were also more likely to report excess nutrient intake for vitamin A, niacin, folate, iron, zinc, and calcium. There were very low percentages (i.e., <5%) of cancer survivors who reported excessive nutrient intake above the UL, with the exceptions of iron (9.4%), niacin (8.4%), zinc (6.3%), and folate (5.1%). To date, no prior studies have evaluated whether excess nutrient intake affects long-term health or behavior outcomes in cancer survivors. However, some evidence suggests that high doses of dietary supplement use may increase cancer risk. Findings from two studies, the Beta-Carotene, and Retinol Efficacy Trial (CARET) and the Alpha-Tocopherol, Beta-Carotene Cancer Prevention (ATBC) study showed increased lung cancer risk among smokers who used beta-carotene supplements at 20 or 30 mg/d29, 30. Likewise, intake of vitamin E (400 IU/d) from supplements increased risk of prostate cancer, as reported by the Selenium and Vitamin E Cancer Prevention Trial (SELECT).31 Moreover, in the Cancer Prevention Study (CPS)-II Nutrition Cohort study, high dose of supplemental calcium intake (≥1000 mg/d) was found to increase the risk of all-cause mortality. However, this positive association was observed among men only and was not statistically significant when lower doses (<1000 mg/d) were used.32 Thus, nutrient intake may affect health outcomes in a U-shape relationship, with both inadequate and excess intake potentially resulting in harms. Both short- and long-term impacts of dietary supplement use on survival outcomes, in particular when high doses are used, have not been clearly elucidated among cancer survivors, which warrant further investigations.
We found that dietary supplement use among childhood cancer survivors was associated with older age, female gender, non-Hispanic white race, higher levels of education and income, insurance coverage, healthcare utilization, and a cluster of healthy lifestyle factors. On the other hand, dietary supplement use was not associated with cancer diagnosis, treatment exposure, and symptom burden. These findings suggest that supplement use is more likely an indicator of high socioeconomic status and overall healthy lifestyle. In addition, dietary supplement use was not associated with physical functioning as assessed by the physical summary score of the HRQOL. This implies that quality of life impairments may not motivate cancer survivors to pursue dietary supplement use among adult survivors of childhood cancer survivors. Instead, survivors with a better mental summary score and those who reported better emotional well-being and higher vitality were more likely to use dietary supplements than those with a lower score.
This study’s strengths included quantification of supplement use in a large heterogeneous cohort of adult survivors of childhood cancer. The availability of treatment, symptom, and HRQOL data allowed us to assess whether exposure to various treatments, presence of multiple symptoms, and HRQOL impairments were associated with dietary supplement use. There were several limitations to this study, including measurement errors associated with self-reported supplement use and dietary intake. However, the validity coefficients for most nutrients assessed using the Block FFQ have been demonstrated by prior validation studies and were considered reasonable.13–15 In addition, to ensure the quality of dietary data, we identified and excluded cohort participants whose dietary intake were potentially unreliable. We adjusted for age, sex, and race/ethnicity when comparing nutrient intake between childhood cancer survivors and community controls although the observed difference could be attributed by some unmeasured factors that differ between the two groups and are associated with nutrient intake. Similarly, when evaluating HRQOL between survivors who used and did not use dietary supplements, we adjusted for a prior list of confounders. However, the residual confounding is likely to still occur as it occurs in most observational studies. Our results could also be subjective to the effect of bias from non-participation and non-response because not all childhood cancer survivors who met the inclusion criteria had participated or had completed questionnaires and clinical assessments. However, we conducted an analysis of the overall SJLIFE cohort, and participants and non-participants did not differ substantially.33 Notably, this was a cross-sectional study. We therefore could not determine when cancer survivors initiated new supplement use (e.g., before or after cancer diagnosis) and the duration of supplement use. Future longitudinal studies may be helpful and needed in identifying whether dietary supplement use impacts on survivors’ long-term symptom and functional outcomes.
Despite these limitations, our study was among the first studies that evaluated dietary supplement use in a group of adult survivors of childhood cancer with large histological diversity and added new knowledge to the current literature. We found that dietary supplement use among childhood cancer survivors was associated with a lower prevalence of inadequate intake as well as a higher prevalence of excess intake of specific nutrients. Findings from this study indicated a need for more careful monitoring of the use of dietary supplement among cancer survivors by their healthcare providers. Future studies are required to evaluate the impact of dietary supplement use on long-term health of childhood cancer survivors.
Supplementary Material
Sources of Support:
This study was supported by NIH/NCI 1R03CA199516-01 (FFZ), NIH/NCI U01CA195547 (MMH & KKN), and by ALSAC. The funding sources had no role in the design, conduct, or analysis of this study or the decision to submit the manuscript for publication.
Footnotes
Conflict of Interest (COI) Statement: The authors have no conflicts of interests to disclose.
REFERENCES
- 1.Zhang FF, Ojha RP, Krull KR, et al. Adult Survivors of Childhood Cancer Have Poor Adherence to Dietary Guidelines. The Journal of Nutrition. Dec 2016;146(12):2497–2505. doi: 10.3945/jn.116.238261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.team TACSmaec. Risks and side effects of dietary supplements. Updated March 31, 2015. Accessed November 15, 2018, 2018. https://www.cancer.org/treatment/treatments-and-side-effects/complementary-and-alternative-medicine/dietary-supplements/risks-and-side-effects.html
- 3.Rock CL, Doyle C, Demark-Wahnefried W, et al. Nutrition and physical activity guidelines for cancer survivors. CA: a cancer journal for clinicians. Jul-Aug 2012;62(4):243–74. doi: 10.3322/caac.21142 [DOI] [PubMed] [Google Scholar]
- 4.Velicer CM, Ulrich CM. Vitamin and mineral supplement use among US adults after cancer diagnosis: a systematic review. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. Feb 01 2008;26(4):665–73. doi: 10.1200/jco.2007.13.5905 [DOI] [PubMed] [Google Scholar]
- 5.Patterson RE, Neuhouser ML, Hedderson MM, Schwartz SM, Standish LJ, Bowen DJ. Changes in diet, physical activity, and supplement use among adults diagnosed with cancer. Journal of the American Dietetic Association. Mar 2003;103(3):323–8. doi: 10.1053/jada.2003.50045 [DOI] [PubMed] [Google Scholar]
- 6.Hudson MM, Ness KK, Gurney JG, et al. Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA : the journal of the American Medical Association. Jun 12 2013;309(22):2371–81. doi: 10.1001/jama.2013.6296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hudson MM, Ness KK, Nolan VG, et al. Prospective medical assessment of adults surviving childhood cancer: study design, cohort characteristics, and feasibility of the St. Jude Lifetime Cohort study. Pediatric blood & cancer. May 2011;56(5):825–36. doi: 10.1002/pbc.22875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Center for Disease Prevention and Control. https://wwwn.cdc.gov/nchs/nhanes/search/datapage.aspx?Component=Dietary&CycleBeginYear=2009. Access on December 18, 2017.
- 9.Rock CL. Multivitamin-multimineral supplements: who uses them? The American journal of clinical nutrition. Jan 2007;85(1):277s–279s. [DOI] [PubMed] [Google Scholar]
- 10.Kwan ML, Greenlee H, Lee VS, et al. Multivitamin use and breast cancer outcomes in women with early-stage breast cancer: the Life After Cancer Epidemiology study. Breast cancer research and treatment. Nov 2011;130(1):195–205. doi: 10.1007/s10549-011-1557-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L. A data-based approach to diet questionnaire design and testing. American journal of epidemiology. Sep 1986;124(3):453–69. [DOI] [PubMed] [Google Scholar]
- 12.U.S. Department of Agriculture. Composition of Foods Raw, Processed, Prepared USDA National Nutrient Database for Standard Reference August 2013. Accessed October 2014. http://www.ars.usda.gov/SP2UserFiles/Place/80400525/Data/SR26/sr26_doc.pdf
- 13.Subar AF, Thompson FE, Kipnis V, et al. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires : the Eating at America’s Table Study. American journal of epidemiology. Dec 15 2001;154(12):1089–99. [DOI] [PubMed] [Google Scholar]
- 14.Block G, Thompson FE, Hartman AM, Larkin FA, Guire KE. Comparison of two dietary questionnaires validated against multiple dietary records collected during a 1-year period. Journal of the American Dietetic Association. Jun 1992;92(6):686–93. [PubMed] [Google Scholar]
- 15.Block G, Woods M, Potosky A, Clifford C. Validation of a self-administered diet history questionnaire using multiple diet records. Journal of clinical epidemiology. 1990;43(12):1327–35. [DOI] [PubMed] [Google Scholar]
- 16.Food and Nutrition Board IoM. Dietary Reference Intakes. 2011.
- 17.Huang IC, Brinkman TM, Kenzik K, et al. Association between the prevalence of symptoms and health-related quality of life in adult survivors of childhood cancer: a report from the St Jude Lifetime Cohort study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. Nov 20 2013;31(33):4242–51. doi: 10.1200/jco.2012.47.8867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waren JE, Kosinski M, Dewey JE. How to scroe version 2 of the SF-36 Health Survey. QualityMetric Incorporation; 2000. [Google Scholar]
- 19.Ainsworth BE, Haskell WL, Herrmann SD, et al. 2011 Compendium of Physical Activities: a second update of codes and MET values. Medicine and science in sports and exercise. Aug 2011;43(8):1575–81. doi: 10.1249/MSS.0b013e31821ece12 [DOI] [PubMed] [Google Scholar]
- 20.Physical Activity Guidelines for Americans (2008).
- 21.2015–2020 Dietary Guidelines for Americans.
- 22.Glickman ME, Rao SR, Schultz MR. False discovery rate control is a recommended alternative to Bonferroni-type adjustments in health studies. Journal of clinical epidemiology. Aug 2014;67(8):850–7. doi: 10.1016/j.jclinepi.2014.03.012 [DOI] [PubMed] [Google Scholar]
- 23.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society. 1995;57(1):289–300. [Google Scholar]
- 24.Comparing the HEI-2015, HEI-2010 & HEI-2005 (2018).
- 25.Miller PE, Vasey JJ, Short PF, Hartman TJ. Dietary supplement use in adult cancer survivors. Oncology nursing forum. Jan 2009;36(1):61–8. doi: 10.1188/09.onf.61-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller P, Demark-Wahnefried W, Snyder DC, et al. Dietary supplement use among elderly, long-term cancer survivors. Journal of cancer survivorship : research and practice. Sep 2008;2(3):138–48. doi: 10.1007/s11764-008-0060-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim Y, Je Y. Vitamin D intake, blood 25(OH)D levels, and breast cancer risk or mortality: a meta-analysis. British journal of cancer. May 27 2014;110(11):2772–84. doi: 10.1038/bjc.2014.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ng K, Nimeri HS, McCleary NJ, et al. SUNSHINE: Randomized double-blind phase II trial of vitamin D supplementation in patients with previously untreated metastatic colorectal cancer. Journal of Clinical Oncology. 2017; [Google Scholar]
- 29.Alpha-Tocopherol BCCPSG. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N Engl J Med. Apr 14 1994;330(15):1029–35. doi: 10.1056/NEJM199404143301501 [DOI] [PubMed] [Google Scholar]
- 30.Omenn GS, Goodman GE, Thornquist MD, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med. May 2 1996;334(18):1150–5. doi: 10.1056/NEJM199605023341802 [DOI] [PubMed] [Google Scholar]
- 31.Klein EA, Thompson IM Jr., Tangen CM, et al. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA : the journal of the American Medical Association. Oct 12 2011;306(14):1549–56. doi: 10.1001/jama.2011.1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang B, Campbell PT, Gapstur SM, et al. Calcium intake and mortality from all causes, cancer, and cardiovascular disease: the Cancer Prevention Study II Nutrition Cohort. The American journal of clinical nutrition. Mar 2016;103(3):886–94. doi: 10.3945/ajcn.115.117994 [DOI] [PubMed] [Google Scholar]
- 33.Ojha RP, Oancea SC, Ness KK, et al. Assessment of potential bias from non-participation in a dynamic clinical cohort of long-term childhood cancer survivors: results from the St. Jude Lifetime Cohort Study. Pediatric blood & cancer. May 2013;60(5):856–64. doi: 10.1002/pbc.24348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Physical Activity Guideline Advisory Committee, Physical Activity Guideline for Americans. Washington DC: US Department of Health and Human Services. 2008; [Google Scholar]
- 35.Farivar SS, Cunningham WE, Hays RD. Correlated physical and mental health summary scores for the SF-36 and SF-12 Health Survey, V.I. Health Qual Life Outcomes. Sep 07 2007;5:54. doi: 10.1186/1477-7525-5-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


