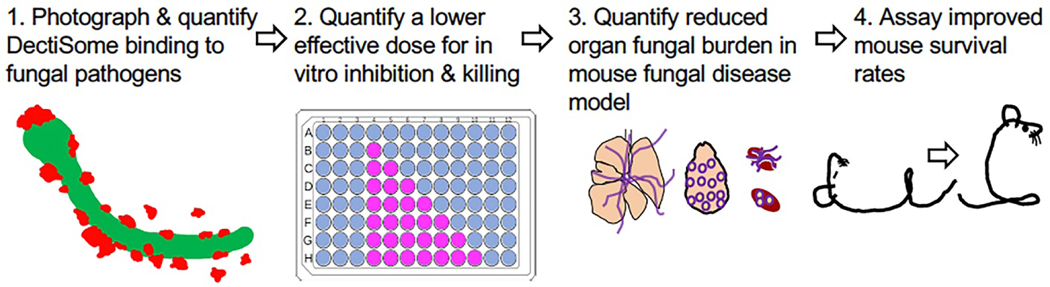
Fig. 3. Validation pipeline to demonstrate the improved efficacy of different classes of DectiSomes against diverse pathogens relative to untargeted antifungals.
Our validation pipeline for DectiSomes proceeds through four steps. (1) We characterize and quantify DectiSome binding to various and diverse fungal pathogens. (2) We quantify the effective dose of antifungal delivered by DectiSomes for inhibition and killing in vitro grown fungal cells. (3) Using mouse models of fungal disease, we quantify the effective dose of antifungal delivered by DectiSomes to reduce fungal burden in target organs. (4) Based on fungal burden experiments, we assay the dose(s) necessary to improve rates of mouse survival. Data are quantified relative to untargeted drug loaded liposomes.