Abstract
Given that pediatric genomic sequencing (GS) may have implications for the health and well-being of both the child and family, a clearer understanding of the key drivers of the utility of GS from the family perspective is needed. The purpose of this study is to explore what is important to caregivers of pediatric patients regarding clinical GS, with a focus on family-level considerations. We conducted semi-structured interviews with caregivers (n = 41) of pediatric patients who had been recommended for or completed GS that explored the scope of factors caregivers considered when deciding whether to pursue GS for their child. We analyzed the qualitative data in multiple rounds of coding using thematic analysis. Caregivers raised important family-level considerations, in addition to those specifically for their child, which included wanting the best chance at good quality of life for the family, the ability to learn about family health, the impact on the caregiver’s well-being, privacy concerns among family members, and the cost of testing to the family. We developed a framework of key drivers of utility consisting of four domains that influenced caregivers’ decision making: underlying values, perceived benefits, perceived risks, and other pragmatic considerations regarding GS. These findings can inform measurement approaches that better capture the utility of pediatric GS for families and improve assessments of the value of clinical GS.
Subject terms: Economics, Health policy, Paediatrics, Health care economics
Introduction
Genomic sequencing (GS), including genome and exome sequencing, may have implications for the health and well-being of the proband and their family. Given the familial nature of genetic information, the severity of many genetic conditions that manifest early in life, and the large role that caregivers may have in lives of children with genetic conditions, implications for families are especially salient in the context of pediatric GS. To guide efficient implementation of genomic services, both health and non-health effects on probands and their family members should be accounted for in assessments of economic value [1, 2].
Direct health effects of GS for probands’ relatives, primarily achieved through cascade screening, may be assessed using measures of health-related quality of life that capture the impacts on changes in medical management and health outcomes. Non-health effects of GS, which are encompassed in the construct of personal utility, include outcomes that patients find meaningful but which do not directly relate to clinical care, such as feelings of control, the value of knowing information, educating oneself, feelings of altruism, and the ability to cope with a clinical condition [3–5]. Non-health effects can be relevant to both the patient and to the family unit.
Parents and caregivers of pediatric patients evaluated for genetic conditions have expressed a desire to understand how GS might impact not only their child but also themselves, other family members such as the proband’s siblings, and potential future children [6]. Parents perceive alleviation of the stress of having an undiagnosed child as a potential benefit of GS [7], and results may make them feel empowered to make informed reproductive decisions and to better communicate about their child’s condition with family members and other individuals who frequently work with their children, such as teachers and caregivers [8].
While non-health effects of a child’s GS on family members have been documented in qualitative research [3, 6–8], the full family-level utility of testing is rarely captured in quantitative evaluations of the value of GS. Comprehensive assessments of family-level utility require valuation of health and non-health outcomes through preference-based methods, which assign values derived from surveys of patients or the public to various outcomes [9]. Although non-health outcomes are not routinely accounted for in economic evaluations [1], recent emphasis on patient-centeredness in health care has spurred increased interest in methods to measure aspects of care that are important to patients. In particular, discrete choice experiments (DCEs) have gained popularity as a method to elicit and quantify preferences in health economics broadly [10–12], and within the context of genetic testing specifically [1, 13]. DCEs are a type of stated preference survey that can be used to measure the relative importance that respondents assign to characteristics, referred to as attributes, of a health care intervention [11]. A critical first step in designing a DCE is conducting formative qualitative research in a target population appropriate for the intervention under study [14–16]. Formative work supporting attribute choice and labeling has received increasing attention [7, 17–19], yet the process of attribute development is infrequently described in detail [20]. Thorough, fit-for-purpose qualitative work can inform preference-based valuation of both health and non-health outcomes, ultimately serving to make economic evaluations more patient-centered by better reflecting what matters to patients and families.
Many studies have explored what parents of children with rare or undiagnosed diseases find important about GS [4, 7, 8, 21, 22], yet no qualitative research has explicitly focused on the value of pediatric GS from a family perspective. The purpose of this study is to explore what is important to parents and caregivers of pediatric patients regarding clinical GS for their child, with a focus on family-level considerations. This formative research and resultant framework will be used to guide the development of attributes for use in a subsequent DCE to enable the quantification of family-level utility for genomic sequencing.
Subjects and methods
Participants
We conducted semi-structured interviews with parents and other primary caregivers (hereafter, “caregivers”) of pediatric patients who were evaluated for a suspected genetic condition at an academic medical center in Texas. To identify potential participants, we reviewed electronic medical records (EMRs) and clinic administrative records on patients recommended for exome sequencing. We used purposive sampling to select potential participants based on their child’s exome result status (recommended to have GS or received GS results within the past year) to ensure that we included a range of perspectives regarding decision making and experienced effects of GS. Individuals were eligible if they met the following criteria: 1) were 18 years of age or older; 2) were a primary caregiver of a living child who had been evaluated for a suspected genetic condition in the general or specialty outpatient genetics clinic; 3) within the past year, their child had either been recommended for exome sequencing or had completed exome sequencing and received results; 4) were fluent in English or Spanish. The contact information for eligible individuals was securely stored in a REDCap database [23].
Via email or text message, we invited eligible individuals to participate in a single, voluntary research interview. Interviews were conducted by telephone or videoconference. All study materials were available in both English and Spanish. Participants were compensated with a $50 electronic gift card. This research was approved by the Baylor College of Medicine Institutional Review Board (H-48379).
Data collection
To inform the development of the interview guide, we reviewed qualitative studies of parental perspectives on GS that were conducted to design preference research [7, 17, 24–26] and articles reporting results of DCEs in genomics and precision medicine. Interviews were conducted in English by a postdoctoral researcher with extensive prior experience in genomics research (HSS) and in Spanish by a Masters-level trained qualitative researcher (AMG) and clinical genomics research coordinator with qualitative research training (AMR), none of whom had a prior relationship with the participants. Interviews were audio recorded, professionally transcribed, checked for accuracy, and de-identified prior to analysis.
We developed two parallel versions of the interview guide, one for caregivers of a child recommended for GS and one for caregivers of a child who had received GS results (Supplementary Information). We asked caregivers about their child and their decision making regarding GS, including how they thought it might impact their family, factors they considered when deciding whether to have the testing, and the most important aspects of having the results (either anticipated or realized). After completion of the interview, we asked participants to respond to a brief online survey administered via REDCap (Supplementary Information). We used novel items to assess the participant’s relationship to the child and the perceived severity of the child’s health condition. The participant’s self-reported general health was assessed using the SF-1 (adapted from the SF-12) [27]. From the EMR, we collected information pertaining to the child’s genetics clinic visit, including a description of their phenotypic presentation, clinical diagnosis, and exome sequencing result.
Analysis
We analyzed qualitative data in multiple rounds of coding using thematic analysis [28]. To develop a coding scheme, three investigators (HSS, SP, ALM) independently derived initial codes and applied them to six transcripts. Using the initial codes, we developed a consensus coding scheme, which two investigators (HSS, ESB) iteratively refined as they applied it to the remaining transcripts and developed themes.
We grouped developed themes into topics and domains, and from the domains we constructed a framework of key drivers of family-level utility. We used MAXQDA [29] and Microsoft Excel to facilitate coding, analysis, and data management. Member checking was not conducted. Reporting of this work adheres to the consolidated criteria for reporting qualitative research (COREQ) and guidelines for reporting formative qualitative research to support the development of quantitative preference studies [30, 31].
Results
We contacted 104 eligible individuals, 59 (57%) of whom responded and agreed to participate and 3 (3%) of whom responded and actively declined participation. Of the 59 individuals who agreed to participate, 41 completed an interview. Interviews were conducted between June 1 and September 1, 2021 and lasted an average of 37 (range: 18–73) minutes.
Participant characteristics
Participants’ children were either recommended for GS (n = 20) or had received GS results within the past year (n = 21). Only one participant whose child had been recommended to receive exome sequencing had chosen not to complete the testing at the time of the interview, as the child’s condition had been satisfactorily diagnosed by chromosomal microarray (CMA). Characteristics of participants and their children are presented in Table 1. Participants’ children presented with a wide range of severity and clinical diagnoses (Supplementary Information), including various developmental delays (n = 14) and multisystem disorders (n = 6). Among 21 GS results returned, eight (38.1%) were diagnostic of the child’s condition.
Table 1.
Interview participant characteristics (n = 41).
| Mean (SD) | |
|---|---|
| Caregiver age | 36.70 (7.78) |
| n (%) | |
| Caregiver relationship to patient | |
| Biological mother | 33 (80.5%) |
| Biological father | 3 (7.3%) |
| Legal guardian | 3 (7.3%) |
| Foster Mother | 1 (2.4%) |
| Stepmother | 1 (2.4%) |
| Caregiver self-reported health | |
| Fair | 5 (12.2%) |
| Good | 13 (31.7%) |
| Very Good | 19 (46.3%) |
| Excellent | 4 (9.8%) |
| Caregiver gender | |
| Female | 37 (90.2%) |
| Male | 4 (9.8%) |
| Caregiver marital status | |
| Married | 32 (78.0%) |
| Divorced | 3 (7.3%) |
| Never married | 3 (7.3%) |
| Living with partner | 3 (7.3%) |
| Caregiver race and ethnicity | |
| Asian | 3 (7.3%) |
| Black or African American | 4 (9.8%) |
| White or European American | 17 (41.5%) |
| Hispanic or Latino | 15 (36.6%) |
| Multiracial | 2 (4.9%) |
| Caregiver education level | |
| High school graduate or less | 10 (24.4%) |
| Some college or Associate’s degree | 10 (24.4%) |
| Bachelor’s degree | 12 (29.3%) |
| Graduate or professional degree | 9 (22.0%) |
| Caregiver household income | |
| Less than $40,000 | 12 (29.3%) |
| $40,000 to $79,999 | 14 (34.1%) |
| $80,000 to $139,999 | 7 (17.1%) |
| $140,000 or more | 8 (19.5%) |
| Child’s insurance provider | |
| Private | 22 (53.7%) |
| Public | 19 (46.3%) |
| Interview language | |
| English | 35 (85.4%) |
| Spanish | 6 (14.6%) |
| Caregiver-reported severity of their child’s health condition | |
| Mild | 10 (24.4%) |
| Moderate | 20 (48.8%) |
| Severe | 11 (26.8%) |
| Child’s exome status at interview | |
| Exome result received | 21 (51.2%) |
| Exome testing submitted | 19 (46.3%) |
| Exome testing recommended but not pursuing | 1 (2.4%) |
Thematic analysis findings
Domains, topics, and themes identified through qualitative analysis are summarized in Fig. 1 and Table 2. Each theme is described more fully in the sections that follow. Developed themes were relevant to both those who were considering GS and those who had completed the testing.
Fig. 1. Framework of key drivers of family-lelve utility of genomic sequencing.
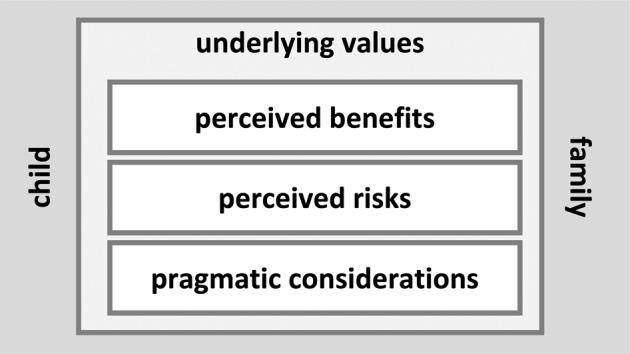
Interview participants considered decisions about pediatric genomic sequencing in the context of their child’s and their family’s well-being, and underlying values shaped how they perceived the benefits, risks, and pragmatic considerations surrounding genomic sequencing.
Table 2.
Summary of thematic analysis findings in four domains relevant to caregivers’ decision making about genomic sequencing.
| Domain | Topic | Theme | Example Quote |
|---|---|---|---|
| Underlying values | Wanting the best chance at good quality of life for the family | Desire for "normal" life and avoidance of suffering | “I think the future of having more children, because we wouldn’t want another baby to go through this, if we could prevent it. I guess that was like our big thing. Like we both said, we want to do genetic testing to know this isn’t something that we’re going to keep passing on.” (ID 114, biological mother, result returned, non-diagnostic) |
| Showing compassion and understanding toward the child | Better understanding and responding to child’s needs | “[…] Understanding hopefully the disability or what it is, understanding on all parts, myself, as a family, as a whole, outsiders, other people, educating others that differences is okay. Sometimes we live in this world today that we notice differences are not always perceived well, so I think that would be a benefit for us and for [child’s name].” (ID 30, biological mother, recommended for GS) | |
| Altruism | Wanting to help child and similarly affected others | “I want to know exactly what is wrong. […] I just want to know so I can better help her, and also so other people, other that have the same condition, would have more information.” (ID 31, biological mother, recommended for GS) | |
| Perceived benefits | Potential to find an answer | Understand the nature of the condition | “Having just maybe hopefully some conclusive answer to what maybe is going on with her and why maybe she was showing these things and is this something that is going to get worse, is it something we can build on and work through and get better?” (ID 12, biological mother, recommended for GS) |
| Understand why and how the condition had come about | “So that kind of just reaffirmed my decisions and try again for another [child]. And also it just reaffirmed that I didn’t do anything ... that it wasn’t anything genetically that I guess that predisposed him to that condition. So it didn’t really definitively tell me much, but it did tell me that it wasn’t anything on our end that we would have to worry about for future I guess.” (ID 102, biological mother, result returned, non-diagnostic) | ||
| Avoid misdiagnosis | “Sometimes I feel like autism isn’t enough for me, enough of an answer. It’s an easy answer because it’s a huge spectrum and there’s all different kinds of autistic kids, but it sometimes feels like why is he so delayed? What’s going on? […] I just want as much information as possible to give him as much as he needs to understand his world.” (ID 37, stepmother, recommended for GS) | ||
| Ability to guide care and pragmatic plans, now or in the future | Hope for targeted therapies | “Biggest benefits would be to know exactly what it is and to figure out how to treat it, if there’s medications available to help treat it. Could therapies help? It depends on what it is, therapies could be beneficial to whatever it is. Not just for us but for him as well.” (ID 68, foster mother, result returned, non-diagnostic). | |
| Avoiding misguided care | “I just look at it the way I am now, the way I live my life. I don’t regret anything, but I do wish I could have done more and I don’t want them to feel that way at all. I want them to have those opportunities and to actually be able to say, "Hey, we did what we could, we did what we were supposed to." And we didn’t just leave them behind in the back like they did with me.” (ID 22, biological mother, recommended for GS). | ||
| Anticipating future life | “Just knowing if he’ll have any continual limitations. Is there a limit to what therapy can do for him? Just that this is as far as he’ll get and that’s what it is, or is there something in the future that we need to be on the lookout for? It is the degenerative side of his genetic stuff [that] scares the living crap out of us. [...] I’m a planner, I’d rather know what we’re coming up against and be on the lookout for it. And take time to set that in versus being surprised because I didn’t want to know. I believe the more information the better.” (ID 61, biological mother, recommended for GS) | ||
| Ability to learn about family health | Implications for family members’ diagnosis | “For us, it won’t change anything. But it could definitely change things in his case and for his brothers, depending on their families and their foster families and if they feel the ability to still care for them, if they were to have a genetic disorder.” (ID 6, legal guardian, recommended for GS) | |
| Impact on caregiver’s wellbeing | Physical consequences of stress | “A week ago I just got the shingles. Often it’s brought on by stress, I’m like so it could be... I definitely think that could be related to this just kind of working with him. It’s not his fault. It’s just I think the nature. I do feel like at least I would feel more at ease if I just knew either that something was going on or I’d be for sure nothing else is going on.” (ID 37, stepmother, recommended for GS) | |
| Emotional burdens | “[...]a lot of scary, worrisome nights and obsessive researching, trying to figure out what’s wrong and just explain, trying to figure it out without thinking that it’s in my head [...] Now that we have everything we need and we are treating him for what he needs to be treated, it’s definitely more relaxing. Not relaxing, but less stressful.” (ID 84, biological mother, result returned, non-diagnostic) | ||
| Ability to open doors | Access to school or community resources | ”[…] having a diagnosis will definitely help her in school because a lot of the times schools, they want that piece of paper for that diagnosis to get them help.” (ID 78, biological mother, result returned, diagnostic) | |
| Perceived risks | Risk of learning psychologically distressing information | Feeling guilt or shame as a parent | “I guess the most riskiest thing is just having guilt of something that you may have caused, but you can’t control it. It’s not actually your fault or anything, but that’s really the only risk in my opinion.” (ID 84, biological mother, result returned, non-diagnostic) |
| Learning information about the parent themselves | “To be 100% honest, I think [child’s dad is] scared. [Child’s name] has scared him his whole life. And his dad has delays as well. Not major delays, but he’s got delays. And I think his dad’s terrified to know what reality is, if there’s something going on. That means, hey, this is wrong with me and it could be wrong with my other kids. […] And he straight up told me, he doesn’t want to know anything. He’s like, ‘I don’t want them to tell me anything.’ And I’m like, ‘Well, that’s your prerogative, if you don’t want to know. I need to know. I need to know if there is something going on that we can help, other than him being a premature baby.’” (ID 49, biological mother, recommended for GS) | ||
| Privacy concerns | Concern about use of genetic information | “No risk that I was concerned about other than the possible where would the information go and what would it be used for. That was probably the only concern that I could think of, the risk.” (ID 87, biological mother, result returned, diagnostic) | |
| Pragmatic considerations | Mode of sample collection | Preference for buccal because less invasive | “It was just saliva. I didn’t see any risks ... I mean, he didn’t like it, but it wasn’t like he has to take blood work. I didn’t really see much of a risk. It’s not like he exposed to needles. It’s not like he has a risk of contamination from anything.” (ID 1, biological mother, recommended for GS). |
| Preference for buccal because more convenient | “[B]ringing it to [the child’s biological parents] was actually better because if they actually had to go somewhere and have [a blood draw] done, I don’t see them doing that.” (ID 11, biological mother, recommended for GS) | ||
| Preference for blood draw because did not want to do buccal swab incorrectly | “I think I probably would have rather gone in because it would have been done faster rather than me doing it at home because like I say, I was just like, ‘Ah ...’ I was kind of anxious about like I don’t want to mess it up...” (ID 102, biological mother, result returned, non-diagnostic) | ||
| Communication with clinical team | Support of genetic counselors | “They were really good about explaining it and everything. They were wonderful. They really broke down so much for me, and they even explained stuff that really, in my opinion, should have been explained better by some other doctors. I mean, they just went above and beyond.” (ID 12, biological mother, recommended for GS) | |
| Wish test had been better explained | “I do not feel that it was explained extensively what it consists of, the test, the sequencing. I think he did his best in trying to explain. […] I waited a year, well, seven years to do this test, we’re so in that journey that we just need a moment to really comprehend what is going on.” (ID 30, biological mother, recommended for GS) | ||
| Need for environment conducive to processing information | “I think she probably did a great job. It was just me. I couldn’t process so much at once.” (ID 6, legal guardian, recommended for GS) | ||
| Wait time for results | Process too lengthy | “It takes too long. I mean, it takes too long for the entire process when the doctor or developmental pediatrician refer for genetics department to make appointment, and order the test, and clarify with health insurance company, and then to get the test done.” (ID 3, biological mother, recommended for GS) | |
| Importance of being patient | “I did not realize it took so long to get the results back. That’s the only thing. But, I’d rather they take their time and get the correct results back than to rush it and then we have to start all over again.” (ID 68, foster mother, results returned) | ||
| Ability to get updates on findings over time | Continued hope for diagnosis | “Yes, it would be very important for us to follow-up on that, if they can study your case, signs and symptoms to compare with other people. If there is similarity between other people or to reach some conclusion, it would be important.” (ID 86, biological mother, result returned, non-diagnostic) | |
| Cost of testing | Decision to pursue hinged on insurance coverage | “Yeah, that’s why we waited to have them look through insurance and see whether or not it would be covered or not. We weren’t going to do anything without finding out if it was covered or not, because it’s expensive.” (ID 1, biological mother, recommended for GS) | |
| Gratitude for insurance coverage | “But thankfully we didn’t have to pay anything and it was covered by the insurance. So we were really grateful that they covered it so we can get our answers.” (ID 72, legal guardian, result returned, non-diagnostic) | ||
| Some amount that would not be willing to pay for additional information | “It’s more based on the doctor, and the insurance comes secondary, because we said, ‘We want to do this if you want us to do this,’ but if it’s $10,000, that’s not worth it to us. You don’t have enough information on it, so I didn’t do anything enough for it to be worth it.” (ID 90, biological mother, result returned, diagnostic) |
Underlying values
Caregivers’ descriptions of their child and their experiences with seeking genetic evaluation illuminated underlying values that they held related to their child and family. While caregivers’ underlying values were distinct from perceptions of GS itself, they guided the way in which caregivers navigated their child’s day-to-day care and diagnostic odyssey, which in turn shaped the way in which they thought about the features (i.e., benefits and risks) of GS. Underlying values served as lenses through which caregivers approached decisions about their child and family, and they often served as motivating factors for pursuing clinical and behavioral care.
Wanting the best chance at good quality of life for the family
Caregivers wanted their child to reach their full potential and be the best version of themself, which was frequently expressed as wanting the child to be able to live a “normal life.” Knowing a genetic reason for some physical or behavioral symptoms was helpful for addressing their child’s needs and improving their quality of life. Caregivers were motivated by considerations of quality of life for their current child(ren), and also for themselves, their family, and potential future children. Biological parents who were considering having more children wanted to avoid seeing future children experience the same struggles that their child was currently experiencing.
Showing compassion and understanding toward the child
Pursuing GS was one way in which caregivers felt like they could help their child, which they saw as a part of their responsibility as a parent. They also acknowledged that GS could give them, their family, and their community deep insight into the mechanism driving their child’s health condition or behavior, providing a means to alleviate misunderstanding, frustration, and bullying. This insight was critical to understanding their child more completely, which was necessary for them to learn how to better care for and show compassion toward their child.
Altruism
Caregivers expressed a sense of community with other families who, currently or in the future, might search for answers about their child’s health in the way that they had. Many had altruistic motivations, understanding that even if GS did not identify a diagnosis for their child, their child’s DNA would contribute to science in such a way that other families in similar situations might benefit in the future.
Perceived benefits
Caregivers described several features of GS as benefits. Some caregivers had pondered what the information they learned through GS might mean for the health of other family members, and caregivers who were used to putting their child’s health care needs before their own reflected on what GS results could mean for their own well-being.
Potential to find an answer
Most participants pursued GS because they were hoping it would help them “find an answer,” in the form of a molecular diagnosis, identifying a “source,” or causal explanation for their child’s health condition. A further benefit of knowing the cause of their child’s health problems was to understand more about the nature of their child’s health concerns, including what they should expect over time and whether the condition might be progressive.
Caregivers also wanted to understand why and how the condition had come about. Interest in understanding whether the condition had been inherited could shape the way parents thought about the possibility of future pregnancies. Many participants were considering having additional children and knowing the cause of their child’s condition could have implications for whether they would try to have another child.
GS was also seen as a comprehensive test that could help rule out genetic causes of what their child was experiencing, avoid misdiagnosis, or unify clinical diagnoses, especially when their child had received multiple clinical diagnoses but no clear explanation of whether or how they fit together. Caregivers sometimes felt as though there was more going on with their child than the clinical diagnosis that they had received could explain and wanted to make sure that they were not missing other signs or symptoms that might be important for understanding their child’s overall health.
Ability to guide care and pragmatic plans, now or in the future
Caregivers saw potential for GS to help guide clinical care or therapies for their child, either at the present time or in the future. For families that were treating multiple symptoms in their child as they arose, having a coordinated treatment plan to address the cause of health concerns was important. For families that received results with no immediate actionability, knowing the genetic cause of their child’s symptoms provided hope that targeted therapies might be available in the future.
Caregivers considered learning how to guide care as doing what was possible (i.e., what they had control over) to help their child. Knowing a diagnosis could enable caregivers to avoid going down a “very bad path” of misguided care, help them understand what a good care plan looked like, and equip them to be an advocate for their child’s health care needs. For example, one mother with a clinical diagnosis of Usher syndrome involving impaired eyesight and hearing, expressed feeling as though she had been “left behind” as a child because of limited attention to her clinical needs and not ever having genetic testing for her condition. When her children developed speech delay and other symptoms similar to her own, she wanted to do all she could to give them opportunities that she did not have to prevent progression.
Participants also described the ability to use GS results to make pragmatic plans and guide expectation setting for their child as valuable, helping them to anticipate what life might look like for their family in several years. However, despite the importance that caregivers assigned to being able to plan for the future, planning for what might occur was often seen as less pressing than current needs, especially for children who presently needed intensive clinical care or therapies.
Ability to learn about family health
Caregivers thought that GS offered an important opportunity to learn about the health of their child’s family, which could be valuable in several ways. First, it could allow families to understand whether conditions that “run in the family” had an identifiable genetic component. Learning that there was a genetic cause could influence reproductive decision making for families who were considering having more children, even if they already knew that members of their family were commonly affected by a particular type of health problem. GS results could also shed light on the cause of symptoms other family members were experiencing. For example, learning her son’s GS results and more about his diagnosis, especially that it was an X-linked condition, helped one mother explain her daughter’s and her own relatively less severe symptoms. Finally, GS could provide valuable information for children who did not live with their biological family or were in the foster care system. It might have legal implications for the child’s foster care case and clinical implications for the child’s biological siblings, particularly if the siblings would not have access to GS themselves.
Impact on caregiver’s well-being
Serving as a primary caregiver for a child with complex clinical needs, with or without a genetic diagnosis, could affect caregivers’ mental and physical health. Caregivers described experiencing stress, and sometimes clinical anxiety or depression, related to their child’s experience with a suspected genetic condition, their role in constantly managing various symptoms or health needs that came up for their child, and looking for a molecular diagnosis for their child. Some caregivers described physical consequences of their stress, such as a case of shingles and self-neglect that resulted in broken teeth, and some newly sought mental health care. Some caregivers anticipated that GS results could not only potentially help relieve the stress, guilt, and blame they felt, but could also bring about new social connections or peace of mind that would improve their overall well-being.
Ability to open doors
Caregivers described GS results as opening doors, allowing access to school or community resources or to disease-specific family groups. Other parents felt confident that having a diagnosis would help them gain access to more specialized services, yet a diagnosis didn’t always make a difference for access to school resources. The potential for social or community resource access was frequently overshadowed by concerns about health and medical care.
Perceived risks
Many caregivers acknowledged concerns that they, their family members, or the public might have about genetic testing to varying degrees. Risks were described in ways that acknowledged the nature and relational aspects of GS data. Caregivers perceived risks as they related to understanding what results would mean for their child and family in both emotional and pragmatic terms.
Risk of learning psychologically distressing information
Caregivers frequently thought of the possibility of learning information that could be psychologically upsetting, or which they were “afraid to hear.” This included learning that their DNA had contributed to their child’s illness, which could lead to feelings of guilt or shame, and that the child might have a condition for which no treatment is available. A few caregivers described feeling mixed emotions when receiving their child’s diagnostic results; they were glad to have an answer about the cause, but confirmation that their child had a genetic condition was often difficult to process as they thought about uncertainties regarding what the child’s future would hold.
Concern about learning potentially psychologically disruptive information extended to information about the parent. Nevertheless, participants typically wanted the information, even secondary findings, regardless of whether it might cause worry. One participant described wanting to know the risk of adult-onset information for her child, even if it could cause more stress or be something else to worry about.
Privacy concerns
While caregivers rarely expressed personal concerns about privacy of their child’s genetic information, some had discussed such concerns with their spouse or the child’s other family members. Among a child’s biological parents, opinions sometimes differed regarding the weight of privacy concerns and whether the parent should agree to submit a sample for testing. Generally, however, caregivers expressed feeling like genetic information was no different than other types of personal information.
Pragmatic considerations
In addition to perceived benefits and risks, caregivers described several additional aspects of the process of GS that were important to them (Table 2), including how the biological sample was collected for testing (typically by either blood draw or buccal swab) and communication and relationship with the genetic counselor or geneticist. Additionally, parents generally enthusiastically endorsed receiving updates on their child’s test results over time, following reanalysis or reclassification.
Cost to the family and insurance coverage of GS were critical to the decision of whether to pursue testing for many families. Several caregivers noted that they would not have been able to do the testing if they had to pay for it out-of-pocket because it would have simply been unaffordable. Others noted that they would have found a way to pay for testing if insurance had not covered it. Many caregivers expressed gratitude for testing being covered by their insurance, feeling that it was a “blessing” to have coverage, while they acknowledged that not everyone would have access to such testing. A few caregivers offered a theoretical amount that would be willing to pay (e.g., $10,000) and above which they would not have been able to pursue testing. Alongside cost considerations for access to testing, caregivers noted the importance of access to specialists at a large, academic children’s hospital. Several had traveled a long distance from their home to pursue a genetic evaluation and testing for their child, and other caregivers noted that their child’s other family members may not have access to testing.
Discussion
In this interview study with caregivers of children who were either recommended for or who had received GS, we identified several family-level considerations that were relevant to caregivers’ decision whether to pursue GS for their child. Underlying values shaped how caregivers described benefits and risks of testing for their child and the family unit. Caregivers saw GS as a mechanism that, through providing information about the basis of their child’s condition, could promote increased understanding of and compassion toward their child among their family and possibly society. Thematic findings did not differ between those who had completed GS and those who had not, which may be attributable to shared experience of caring for a child with special health care needs.
The potential to “find an answer” was a common perceived benefit of GS, aligned with findings from previous qualitative research to inform patient preference studies on this topic that have been conducted outside of the US. Abbott and colleagues, who conducted interviews with parents of children and adults with undiagnosed rare disease who were participating in the Scottish Genomes Partnership study, also noted repeated instances of this phrase “having an answer” when asking participants what features of GS were important to them [18]. Similarly, in mixed-methods research with various stakeholders in Australia, Best and colleagues found that the likelihood of getting an answer from GS was the most important across stakeholder groups [17]. Our analysis also revealed that neither benefits nor risks were limited to the patient alone. Caregivers’ and family well-being could also be impacted through the child’s GS, and concerns about privacy and learning psychologically distressing information were relevant to parents and caregivers. Unsurprisingly, cost to the family and insurance coverage of GS featured prominently in caregivers’ decision making, with several participants noting that they would not have been able to get the testing without insurance coverage or financial assistance.
Even though submission of samples for GS was not a requirement for participation in this study, only one participant declined to pursue GS after it was recommended. Perspectives of caregivers who choose not to complete recommended testing for their child are important to understand the range of important considerations in families’ decision making about GS. However, because it is rare for families to decline GS and families who do decline are not easily identifiable through EMR review, there are logistical challenges associated with contacting them for research participation. We recruited participants from a single clinic, although it is one of the largest specialty pediatric health care organizations in the US with a genetics clinic that serves more than 3000 families per year and has 18 medical geneticists and 14 genetic counselors [32]. Because we only recruited caregivers of children who were alive to reduce potential discomfort from participating in a research interview, our analysis is limited by not including perspectives of caregivers whose child had died after consideration or completion of GS. Given that only six of the 41 interviews were conducted in Spanish, we did not explore whether themes differed by interview language.
Effects on family members, which have traditionally been a focus of research on the ethical, legal, and social implications of genomics, should be incorporated into assessments of economic value. Our framework of key drivers of utility of GS from a family perspective will inform the design of a subsequent DCE. Future research will explore ways to best expand evaluations of GS along two dimensions: integrating non-health, personal utility outcomes alongside health outcomes, and moving from a focus on the patient to the family.
Supplementary information
Family-level utility of GS - Supplemental Material
Author contributions
HSS confirms that she had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors gave final approval of this version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
Dr. Smith is supported by the National Human Genome Research Institute of the National Institutes of Health under Award Number K99HG011491. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Ethical approval
Approval to conduct this human subjects research was obtained by the Baylor College of Medicine Institutional Review Board. Verbal informed consent was obtained from all participants for being included in the study.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41431-022-01245-0.
References
- 1.Regier DA, Weymann D, Buchanan J, Marshall DA, Wordsworth S. Valuation of Health and Nonhealth Outcomes from Next-Generation Sequencing: Approaches, Challenges, and Solutions. Value Health. 2018;21:1043–7. doi: 10.1016/j.jval.2018.06.010. [DOI] [PubMed] [Google Scholar]
- 2.Smith HS, McGuire AL, Wittenberg E, Lavelle TA. Family-level impact of genetic testing: integrating health economics and ethical, legal, and social implications. Personalized Med. 2021;18:209–12.. doi: 10.2217/pme-2021-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kohler JN, Turbitt E, Biesecker BB. Personal utility in genomic testing: a systematic literature review. Eur J Hum Genet. 2017;25:662–8. doi: 10.1038/ejhg.2017.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mollison L, O’Daniel JM, Henderson GE, Berg JS, Skinner D. Parents’ perceptions of personal utility of exome sequencing results. Genet Med. 2020;22:752–7. doi: 10.1038/s41436-019-0730-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kohler JN, Turbitt E, Lewis KL, Wilfond BS, Jamal L, Peay HL, et al. Defining personal utility in genomics: A Delphi study. Clin Genet. 2017;92:290–7. doi: 10.1111/cge.12998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watnick D, Odgis JA, Suckiel SA, Gallagher KM, Teitelman N, Donohue KE, et al. "Is that something that should concern me?": a qualitative exploration of parent understanding of their child’s genomic test results. HGG Adv. 2021;2:100027. [DOI] [PMC free article] [PubMed]
- 7.Pollard S, Weymann D, Dunne J, Mayanloo F, Buckell J, Buchanan J, et al. Toward the diagnosis of rare childhood genetic diseases: what do parents value most? Eur J Hum Genet. 2021;29(10):1491–501. [DOI] [PMC free article] [PubMed]
- 8.Lee W, Luca S, Costain G, Snell M, Marano M, Curtis M, et al. Genome sequencing among children with medical complexity: What constitutes value from parents’ perspective? J.J Genet Couns. 2022;31:523-33. [DOI] [PubMed]
- 9.Neumann PJ, Goldie SJ, Weinstein MC. Preference-based measures in economic evaluation in health care. Annu Rev Public Health. 2000;21:587–611. doi: 10.1146/annurev.publhealth.21.1.587. [DOI] [PubMed] [Google Scholar]
- 10.Soekhai V, de Bekker-Grob EW, Ellis AR, Vass CM. Discrete choice experiments in health economics: past, present and future. PharmacoEconomics. 2019;37:201–26. doi: 10.1007/s40273-018-0734-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ryan M, Gerard K, Amaya-Amaya M. Using discrete choice experiments to value health and health care: Springer Science & Business Media; 2007.
- 12.Mühlbacher A, Johnson FR. Choice experiments to quantify preferences for health and healthcare: state of the practice. Appl health Econ health policy. 2016;14:253–66. doi: 10.1007/s40258-016-0232-7. [DOI] [PubMed] [Google Scholar]
- 13.Ozdemir S, Lee JJ, Chaudhry I, Ocampo RRQ. A Systematic Review of Discrete Choice Experiments and Conjoint Analysis on Genetic Testing. Patient - Patient-Centered Outcomes Res. 2022;15:39–54. doi: 10.1007/s40271-021-00531-1. [DOI] [PubMed] [Google Scholar]
- 14.Coast J, Horrocks S. Developing attributes and levels for discrete choice experiments using qualitative methods. J health Serv Res Policy. 2007;12:25–30. doi: 10.1258/135581907779497602. [DOI] [PubMed] [Google Scholar]
- 15.Coast J, Al‐Janabi H, Sutton EJ, Horrocks SA, Vosper AJ, Swancutt DR, et al. Using qualitative methods for attribute development for discrete choice experiments: issues and recommendations. Health Econ. 2012;21:730–41. doi: 10.1002/hec.1739. [DOI] [PubMed] [Google Scholar]
- 16.Kløjgaard ME, Bech M, Søgaard R. Designing a stated choice experiment: the value of a qualitative process. J Choice Model. 2012;5:1–18. doi: 10.1016/S1755-5345(13)70050-2. [DOI] [Google Scholar]
- 17.Best S, Stark Z, Phillips P, Wu Y, Long JC, Taylor N, et al. Clinical genomic testing: what matters to key stakeholders? Eur J Hum Genet. 2020;28:866–73.. doi: 10.1038/s41431-020-0576-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abbott M, McKenzie L, Moran BVG, Heidenreich S, Hernández R, Hocking-Mennie L, et al. Continuing the sequence? Towards an economic evaluation of whole genome sequencing for the diagnosis of rare diseases in Scotland. J Commun Genet. 2022;13:487–501. [DOI] [PMC free article] [PubMed]
- 19.Hammond J, Klapwijk JE, Riedijk S, Lou S, Ormond KE, Vogel I, et al. Assessing women’s preferences towards tests that may reveal uncertain results from prenatal genomic testing: Development of attributes for a discrete choice experiment, using a mixed-methods design. PLoS One. 2022;17:e0261898. doi: 10.1371/journal.pone.0261898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vass C, Rigby D, Payne K. The Role of Qualitative Research Methods in Discrete Choice Experiments. Med Decis Mak. 2017;37:298–313. doi: 10.1177/0272989X16683934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith HS, Morain SR, Robinson JO, Canfield I, Malek J, Rubanovich CK, et al. Perceived Utility of Genomic Sequencing: Qualitative Analysis and Synthesis of a Conceptual Model to Inform Patient-Centered Instrument Development. The Patient - Patient-Centered Outcomes Research. 2021. [DOI] [PMC free article] [PubMed]
- 22.Halley MC, Young JL, Fernandez L, Kohler JN, Bernstein JA, Wheeler MT, et al. Perceived utility and disutility of genomic sequencing for pediatric patients: Perspectives from parents with diverse sociodemographic characteristics. Am J Med Genet A. 2022;188:1088–101.. doi: 10.1002/ajmg.a.62619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inf. 2009;42:377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chassagne A, Pélissier A, Houdayer F, Cretin E, Gautier E, Salvi D, et al. Exome sequencing in clinical settings: preferences and experiences of parents of children with rare diseases (SEQUAPRE study) Eur J Hum Genet. 2019;27:701–10.. doi: 10.1038/s41431-018-0332-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lewis MA, Stine A, Paquin RS, Mansfield C, Wood D, Rini C, et al. Parental preferences toward genomic sequencing for non-medically actionable conditions in children: a discrete-choice experiment. Genet Med. 2018;20:181–9. doi: 10.1038/gim.2017.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marshall DA, MacDonald KV, Heidenreich S, Hartley T, Bernier FP, Gillespie MK, et al. The value of diagnostic testing for parents of children with rare genetic diseases. Genet Med. 2019;21:2798–806.. doi: 10.1038/s41436-019-0583-1. [DOI] [PubMed] [Google Scholar]
- 27.Stewart AL, Hays RD, Ware JE., Jr The MOS short-form general health survey. Reliability and validity in a patient population. Med Care. 1988;26:724–35. doi: 10.1097/00005650-198807000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Braun V, Clarke V. Thematic analysis. APA handbook of research methods in psychology, Vol 2: Research designs: Quantitative, qualitative, neuropsychological, and biological. APA handbooks in psychology®. Washington, DC, US:, American Psychological Association; 2012. p. 57-71.
- 29.VERBI Software MAXQDA 2022 Berlin, Germany: VERBI Software; 2021.
- 30.Hollin IL, Craig BM, Coast J, Beusterien K, Vass C, DiSantostefano R, et al. Reporting Formative Qualitative Research to Support the Development of Quantitative Preference Study Protocols and Corresponding Survey Instruments: Guidelines for Authors and Reviewers. The Patient - Patient-Centered Outcomes. Research. 2020;13:121–36.. doi: 10.1007/s40271-019-00401-x. [DOI] [PubMed] [Google Scholar]
- 31.Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care. 2007;19:349–57. doi: 10.1093/intqhc/mzm042. [DOI] [PubMed] [Google Scholar]
- 32.Texas Children’s Hospital Genetics Clinic 2020 [Available from: https://www.texaschildrens.org/departments/genetics.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Family-level utility of GS - Supplemental Material
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


