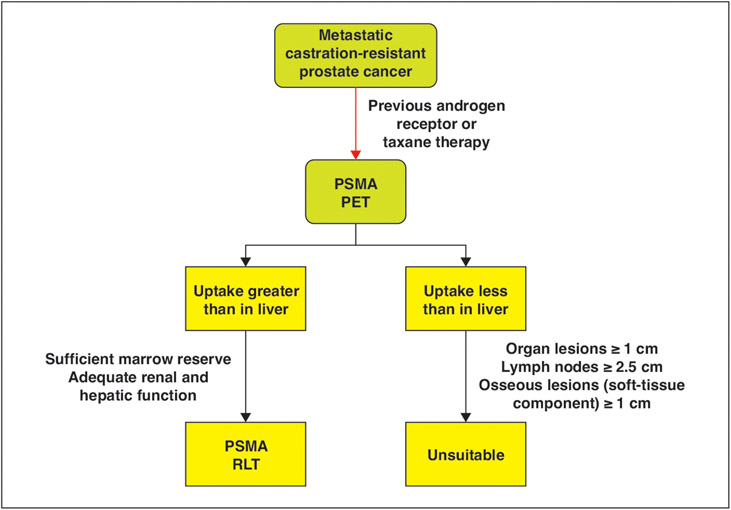
Fig. 4—
Schematic shows overview of 177Lu-labeled PSMA ligands. Eligible patients include those with metastatic castration-resistant prostate cancer previously treated with androgen receptor inhibitors or taxane-based therapy. Patients subsequently undergo PSMA PET. After multidisciplinary discussion, PSMA radioligand therapy (RLT) should be considered in patients with evidence of PSMA uptake greater than liver uptake, sufficient marrow reserve, and adequate renal and hepatic function. PSMA RLT should not be considered in patients with measurable disease (including organ lesions measuring ≥ 1 cm, lymph nodes measuring ≥ 2.5 cm, and bony lesions measuring ≥ 1 cm) that lack PSMA uptake greater than that of liver.