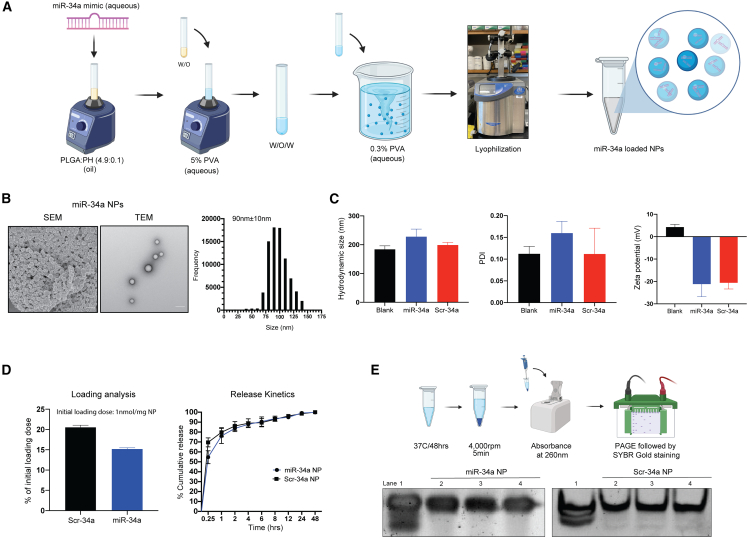
Figure 1.
Formulation of PLGA-poly-L-His-miR-34a nanoparticles containing miR-34a mimic and their biophysical characterization
(A) Schematic showing PLGA-poly-L-His (PH) NP formulation using double emulsion solvent evaporation. PLGA and poly-L-histidine (PLGA-poly-L-His) were solubilized at a 4.9/0.1 w/w ratio in acetone:DCM (2:1) organic solvent. (B) SEM and TEM images of miR-34a-loaded PLGA-PH NPs. The respective size distribution was calculated from the SEM image using ImageJ software. The scale bar represents 100 nm. (C) NP hydrodynamic size (in nm), polydispersity index (PDI), and surface charge density (in mV) of blank, miR-34a, and Scr-34a NPs. Data represent the mean of three batches (n = 3) ± standard deviation. Data for individual batches are shown in Figure S1. (D) Loading (percentage of initial loading/mg NP) and release kinetics as percentage cumulative release of miR-34a and Scr-34a NPs in phosphate-buffered saline. Data represent the mean (n = 3) ± standard deviation. (E) In vitro release analysis to determine the miRNA mimic integrity using polyacrylamide gel electrophoresis. (Top) Schematic showing the workflow for release of miRNA mimic from NPs followed by PAGE. (Bottom) Gel shift assay to analyze the stability of released miRNA mimic stability. Lane 1 contains the miRNA mimic stock at 1 μM concentration. Lanes 2–4 contain the released miRNA mimic (equivalent concentration) from the NPs. Data show samples from three separate NP batches.