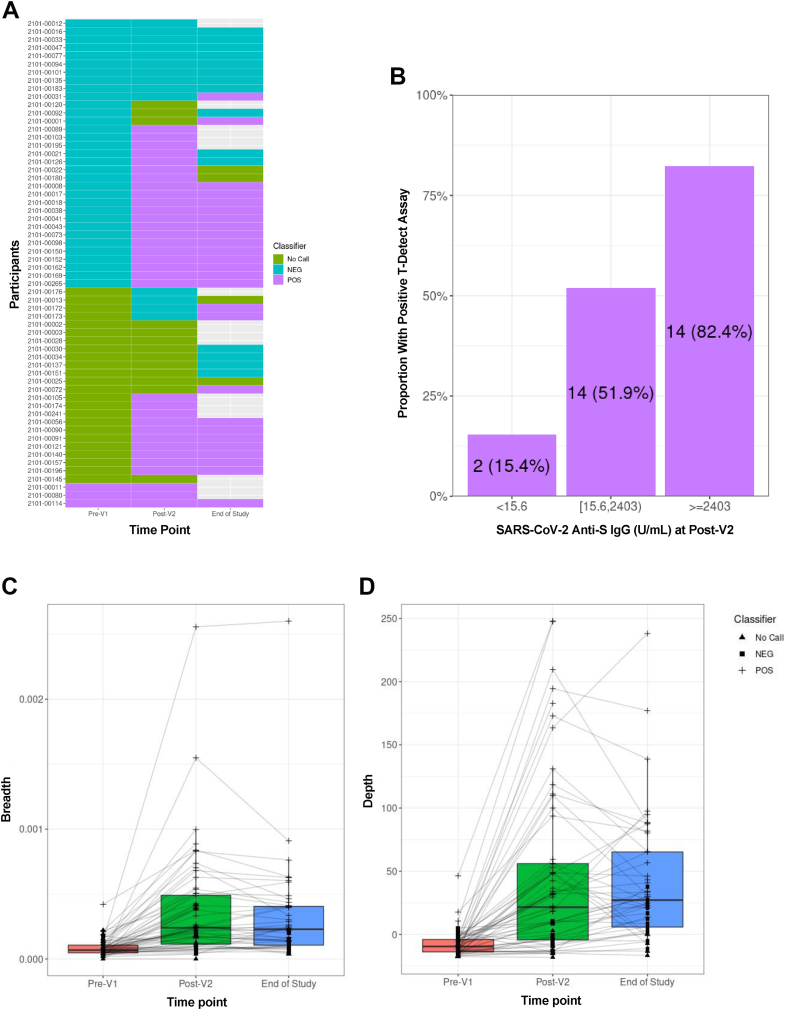
Fig. 2.
SARS-CoV-2-specific T-cell receptor (TCR) variable beta chain sequencing results in a subgroup of 60 participants vaccinated <4 months (n = 19) or 4–12 months (n = 41) after allogeneic HCT. A) Qualitative results indicating a positive, negative, or indeterminate result for the presence of SARS-CoV-2-specific TCRs based on the T-Detect ImmunoSEQ Assay classifier (Adaptive Biotechnologies, Seattle, WA). Each row indicates a unique participant. Unfilled cells at the end-of-study time point indicate that no sample was available for testing. B) The proportion of participants with a positive T-Detect at the post-V2 timepoint in categories of negative (n = 13), any detectable (n = 27), or positive (n = 17) SARS-CoV-2 anti-S IgG titers; three individuals with a positive T-Detect at the pre-V1 time point were excluded. (C and D) Quantitative values at each time point indicating the SARS-CoV-2 TCR breadth (C), defined as the proportion of total unique TCRs associated with SARS-CoV-2; and depth (D), defined as the extent to which SARS-CoV-2-associated TCRs expand.