This cross-sectional study examines whether the emergence of borderline personality disorder is associated with the prioritization of immediate reproductive goals over longer-term somatic maintenance goals.
Key Points
Question
Is borderline personality disorder (BPD) favored by prioritizing immediate reproductive goals over long-term somatic maintenance goals in response to early life adversity?
Findings
In this cross-sectional study of more than 30 000 adults, the association of early life adversity with the risk of being diagnosed with BPD later in life was significantly mediated by an allocation trade-off favoring immediate reproduction over somatic maintenance.
Meaning
BPD may be the psychobehavioral expression of a broader coping strategy whereby individuals deal with adversity by prioritizing the development of reproductive traits and behaviors.
Abstract
Importance
Borderline personality disorder (BPD) is often accompanied by a history of high-risk sexual behavior and somatic comorbidities. Yet, these features are most often considered in isolation and little is known about their underlying developmental pathways. Life history theory, a leading framework in evolutionary developmental biology, can help make sense of the wide range of behaviors and health issues found in BPD.
Objective
To examine whether the emergence of BPD is associated with the prioritization of immediate reproductive goals over longer-term somatic maintenance goals, a life strategy that can be viewed as a developmental response to adverse early life experiences, providing rapid reproductive benefits despite costs to health and well-being.
Design, Setting, and Participants
This study used cross-sectional data from the second wave of the National Epidemiologic Survey on Alcohol and Related Conditions in 2004-2005 (n = 34 653). Civilian, noninstitutionalized individuals in the US, 18 years or older, and those with and without a DSM-IV diagnosis of BPD were included. Analysis took place between August 2020 and June 2021.
Main Outcomes and Measures
Structural equation models were used to examine whether early life adversity was associated with the likelihood of a BPD diagnosis, either directly or indirectly through a life strategy whereby individuals trade somatic maintenance for immediate reproduction.
Results
Analyses were performed on a sample of 30 149 participants (females: 17 042 [52%]; mean [SE] age, 48.5 [0.09]; males: 12 747 [48%]; mean [SE] age, 47 [0.08]). Of these, 892 (2.7%) had a diagnosis of BPD and 29 257 (97.3%) did not have BPD. Mean early life adversity, metabolic disorder score, and body mass index were significantly higher among participants with a diagnosis of BPD. In an analysis adjusted for age, individuals with BPD reported having significantly more children than those without BPD (b =0.06; SE, 0.01; t = 4.09; P < .001). Having experienced greater levels of adversity in early life was significantly associated with a greater risk of being diagnosed with BPD later in life (direct relative risk = 0.268; SE, 0.067; P < .001). Importantly, this risk was further increased by 56.5% among respondents who prioritized short-term reproductive goals over somatic maintenance (indirect relative risk = 0.565; SE, 0.056; P < .001). Similar patterns of associations were found in male and female individuals.
Conclusions and Relevance
The hypothesis of a reproduction/maintenance life history trade-off mediating the association between early life adversity and BPD helps make sense of the high dimensionality that characterizes the physiological and behavioral correlates of BPD. Additional studies are needed to confirm these results using longitudinal data.
Introduction
Borderline personality disorder (BPD) emerges by late adolescence or early adulthood and is highly prevalent in clinical and community samples.1,2 It includes a wide range of symptoms, from unstable interpersonal relationships, fear of abandonment, emotion dysregulation, feelings of emptiness, and chronic dysphoria or depression. These symptoms are often accompanied by behaviors that significantly impair psychosocial functioning such as substance use,3,4 sexual risk-taking,5,6,7 low prosociality,8,9 interpersonal violence, as well as self-harm,5,10,11,12 including suicide attempts.13,14 In addition, individuals with BPD have greater prevalence of somatic comorbidity than individuals with any other personality disorders, which contributes to a shortened life span.2,15,16 The co-occurrence of these seemingly unrelated manifestations makes BPD not well understood.
Substantial effort has been made to delineate the developmental origins of BPD and better identify its early environmental determinants to aid its prevention. It is commonly accepted that BPD is partly rooted in early life, via exposure to adverse events that include all forms of instability, deprivation, neglect, abuses, or violence occurring within or outside the family household.17,18,19 Despite these important advances, little is known about who is at risk of developing BPD.17
To better characterize this risk requires consideration of each individual's ability to respond to early life experiences through developmental changes.20,21 There is evidence of an association between the mortality risks present in an environment and variations in how individuals organize their life cycles to optimize achievement of their priority biological goals, ie, growth and maintenance, social goals, and reproductive goals,22 given limited energetic resources.23 From a biological perspective, it makes sense for organisms to outweigh such risks by allocating more resources to the development of behavioral traits that provide rapid reproductive benefits and fewer resources to somatic maintenance traits that provide longer-term survival benefits.24
The existing literature indicates that individuals with BPD may exhibit such trade-off in a way that does not involve conscious decision-making. Thus, they enter sexual life at a younger age than individuals without BPD, have more sexual partners, more unprotected sex, and, for women in particular, become parents at a younger age and experience more unintended pregnancies,3,5,6,25,26,27 a set of behaviors that is often accompanied by adverse health effects and general medical comorbidities.16,28,29
These observations, viewed through the lens of life history theory, a major framework in evolutionary developmental biology, suggest that BPD may facilitate reproductive goals that provide immediate fitness benefits30,31 and that it may develop to counteract risks estimated from early life experiences.32 Therefore, we tested 3 complementary hypotheses: (1) somatic maintenance traits and short-term reproductive behaviors are negatively correlated through a latent factor representing the resource allocation trade-off described above; (2) adversity experienced early in life is associated with increased risk of BPD expression in adulthood; and (3) the association of early life adversity with the risk of developing BPD is exacerbated for individuals who trade somatic maintenance for short-term reproductive goals. To this end, we used structural equation models (SEMs) coupled with k-fold cross-validation analyses on a large nationally representative sample, the National Epidemiological Survey on Alcohol and Related Conditions (NESARC).
Methods
This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.33
Sample
We used data drawn from the wave 2 NESARC. The wave 1 NESARC (2001-2002) is a representative face-to-face survey that includes 43 093 adult residents of households or group quarters in the US, conducted by the National Institute on Alcoholism and Alcohol Abuse and described in detail elsewhere.34,35 The wave 2 survey (2004-2005) includes 86.7% of the original sample, corresponding to 34 653 completed interviews.35 The wave 2 NESARC data were weighted to be representative of the US civilian population based on the 2000 census.35 The research protocol, including written informed consent procedures, received full human subjects review and approval from the US Census Bureau and the Office of Management and Budget.36 The present study was conducted with this sample of 34 653 adults.
Early Life Adversity
Early life adversity was modeled as a sum of z scores (scaled from 0.0 to 1.0) obtained on 53 items covering factors known to represent the general quality of the respondents’ early environment,37,38 between birth and age 18 years (eMethods 1 in Supplement 1).
BPD
In the NESARC wave 2 interview, all participants were asked about lifetime BPD symptoms. These symptoms were assessed using the National Institute on Alcoholism and Alcohol Abuse Alcohol Use Disorder and Associated Disabilities Interview Schedule-IV, DSM-IV version.39 Analyses for the present study focused on the 9 DSM-IV BPD symptoms (eTable 1 in Supplement 1).40 In line with previous NESARC studies focusing on BPD, we only included symptoms causing social or occupational dysfunction.41,42,43
Reproduction/Maintenance Trade-off
The trade-off meant to arbitrate the allocation of resources between short-term reproduction and somatic maintenance was modeled as a latent factor aiming at capturing the shared variance of 7 indicators commonly reported in human life history research.38,44,45,46,47 The reproductive indicators included the respondents’ number of children, the number of marriages, their age at first sexual intercourse, and history of sexually transmitted disease. The somatic maintenance indicators included the respondents’ body mass index at the time of the interview, their perceived physical health, and their metabolic risk factor (eMethods 2 in Supplement 1). A confirmatory factor analysis confirmed that our reproduction/maintenance trade-off latent factor correlated positively with the participants’ reproductive goals and negatively with their somatic maintenance traits (eFigure 1, eTable 2, and eTable 3 in Supplement 1). Higher scores on the latent factor positively correlated with immediate reproduction and negatively correlated with self-reported health status.
Covariates
All models were adjusted for sex, age, and race (White vs non-White [Black, American Indian/Alaska Native, Asian/Native Hawaiian/Other Pacific Islander, or Hispanic]). Race was categorized as White vs non-White because members of minority racial and ethnic groups (ie, non-White) may experience an overall higher level of environmental adversity, which is a potential confounding factor in our models.75,76,77 All participants were asked to describe their race by selecting 1 or more categories defined by the investigator.
Descriptive Statistics
We calculated the means for continuous variables, the proportions for binary variables, and standard errors among participants with BPD, participants without BPD, and in the full sample. All summary statistics and tests for between-group comparison of each variable took into account the sampling weights and design effects of the NESARC. Descriptive analyses were conducted using the survey R package48 with R software version 3.6.1 (R Foundation) and are summarized in the Table and eTables 2 and 4 in Supplement 1.
Table. Summary Statistics for the National Epidemiological Survey on Alcohol and Related Conditions Variables of Interesta.
| Characteristic | Total (N = 30 149) | No BPD (n = 29 257) | BPD (n = 892) | P value | Cohen d | |||
|---|---|---|---|---|---|---|---|---|
| Mean (SE) | Median (IQR) [range] | Mean (SE) | Median (IQR) [range] | Mean (SE) | Median (IQR) [range] | |||
| Female, % (SE) | 52 (3.72 × 10−5) | NA | 52 (3.77 × 10−5) | NA | 57 (2.23 × 10−4) | NA | <.001 | 0.10 |
| Male, % (SE) | 48 (5.40 × 10−5) | NA | 48 (5.42 × 10−5) | NA | 43 (3.40 × 10−4) | NA | <.001 | 0.10 |
| Age, y | 47.78 (0.06) | 46.00 (34.00-59.00) [20.00-90.00] | 47.98 (0.06) | 46.00 (34.00-59.00) [20.00-90.00] | 40.54 (0.28) | 40.00 (29.00-50.00) [20.00-90.00] | <.001 | 0.44 |
| Race and ethnicity, % (SE) | ||||||||
| American Indian/Alaska Native | 2.2 (7.36 × 10−5) | NA | 2.2 (7.46 × 10−5) | NA | 3.8 (4.42 × 10−4) | NA | <.001 | 0.11 |
| Asian/Native Hawaiian/Other Pacific Islander | 4.0 (7.29 × 10−5) | NA | 4.1 (7.39 × 10−5) | NA | 1.8 (4.5 × 10−4) | NA | <.001 | 0.12 |
| Black | 11 (7.01 × 10−5) | NA | 11 (7.11 × 10−5) | NA | 14 (4.2 × 10−4) | NA | <.001 | 0.10 |
| Hispanic | 12 (7.0 × 10−5) | NA | 12 (7.09 × 10−5) | NA | 11 (4.3 × 10−4) | NA | .003 | 0.03 |
| White | 71 (3.37 × 10−5) | NA | 71 (3.42 × 10−5) | NA | 70 (2.07 × 10−4) | NA | .15 | NA |
| Age at first sexual intercourse, y | 18.26 (0.01) | 18.00 (16.00-20.00) [6.00-78.00] | 18.33 (0.01) | 18.00 (16.00-20.00) [6.00-78.00] | 16.04 (0.08) | 16.00 (14.00-18.00) [6.00-45.00] | <.001 | 0.60 |
| No. of children | 2.14 (8.60 × 10−3) | 2.00 (1.00-3.00) [0-15.00] | 2.14 (8.65 × 10−3) | 2.00 (1.00-3.00) [0-15.00] | 2.06 (0.06) | 2.00 (0-3.00) [0-15.00] | <.001 | 0.04 |
| No. of marriages | 1.08 (3.40 × 10−3) | 1.00 (1.00-1.00) [0-14.00] | 1.08 (3.42 × 10−3) | 1.00 (1.00-1.00) [0-14.00] | 1.17 (0.02) | 1.00 (0-2.00) [0-8.00] | .60 | NA |
| History of sexually transmitted disease, % (SE) | 0.6 (5.62 × 10−6) | NA | 0.5 (5.26 × 10−6) | NA | 3.8 (8.64 × 10−5) | NA | <.001 | 0.43 |
| Perceived physical health | 50.51 (0.04) | 54.50 (47.30-57.50) [4.30-74.30] | 50.58 (0.04) | 54.50 (47.56-57.50) [4.30-74.30] | 47.96 (0.20) | 51.65 (39.78-57.90) [13.30-70.00] | <.001 | 0.25 |
| Body mass indexb | 27.61 (0.02) | 26.61 (23.62-30.54) [8.86-87.43] | 27.59 (0.02) | 26.58 (23.62-30.52) [8.86-87.43] | 28.31 (0.14) | 27.18 (23.49-31.96) [12.21-55.13] | <.001 | 0.12 |
| Metabolic risk factors | 0.81 (3.60 × 10−3) | 1.00 (0-1.00) [0-4.00] | 0.80 (3.55 × 10−3) | 1.00 (0-1.00) [0-4.00] | 0.96 (0.02) | 1.00 (0-2.00) [0-4.00] | <.001 | 0.16 |
| Early life adversity | 0.08 (3 × 10−4) | 0.04 (0.02-0.10) [0-1.00] | 0.07 (3.01 × 10−4) | 0.04 (0.02-0.10) [0-1.00] | 0.20 (3.45 × 10−3) | 0.16 (0.08-0.27) [0-0.91] | <.001 | 1.40 |
Abbreviations: BPD, borderline personality disorder; NA, not applicable.
χ2 Test with Rao-Scott second-order correction and Wilcoxon rank-sum test for complex survey samples was used.
Body mass index was calculated as weight in kilograms divided by height in meters squared.
Structural Equation Models
Structural equation models were used as our main multivariate analysis method. This analysis was conducted using Mplus version 8.1 (Muthén & Muthén).49 Model parameters estimation was conducted using mean- and variance-adjusted weighted least squares estimator.49 We then examined measures of goodness of fit, including the root mean squared error of approximation, the comparative fit index, the Tucker-Lewis index, and the standardized root mean square residual statistics. Root mean square error of approximation values less than 0.05, comparative fit index and Tucker-Lewis index values more than 0.95, and standardized root mean square residual values less than 0.08, which are commonly used to indicate good model fit, were used as cutoffs.
We evaluated a latent mediation model in which (1) the reproduction-maintenance trade-off latent factor was regressed on the early life adversity variable; (2) the BPD diagnostic was regressed on both the reproduction-maintenance trade-off latent factor; and (3) the early life adversity variable. Evidence for mediation of the link between adversity in early life and BPD diagnosis in adulthood by the latent reproductive/maintenance trade-off factor was assessed by 3 complementary analyses (eMethods 3 in Supplement 1). Analysis took place between August 2020 and June 2021.
Model Variations Across Sex
We further evaluated variation of the latent mediation model as well as the variation in the size of its estimates between male and female individuals. For this analysis, we compared nested models and tested differences between them with a robust χ2 difference for mean- and variance-adjusted weighted least squares estimators (eMethods 4 in Supplement 1).50
Sensitivity Analyses and k-Fold Cross-Validation
Several sensitivity analyses were conducted to examine the robustness of the results (eMethods 5 in Supplement 1). Risks of overfitting were estimated by applying a 10-fold cross-validation procedure on each model (eMethods 6 in Supplement 1).51,52,53
Results
Analyses were run on a sample of 30 149 (87% of the initial sample) obtained after listwise deletion of participants with missing variables. The Table provides details of the summary statistics of each variable of interest, as well as the test of their difference between the group of participants with a DSM-IV BPD diagnosis (892 [3%]) and the group of participants without BPD (29 257 [97%]). Mean early life adversity, metabolic disorder score, and body mass index were significantly higher among participants with a diagnosis of BPD. The percentage of female individuals and the percentage of history of sexually transmitted disease in the year prior to the interview were also higher in the BPD subsample. Conversely, mean age at the time of the interview, age at first sexual intercourse, and physical health were all significantly lower among participants with BPD. The mean number of children was also significantly lower among participants with BPD. However, when age was adjusted for in a log-linear regression with number of children as the dependent variable, subsample (BPD vs non-BPD) as the predictor, and age of respondent as the covariate, individuals with BPD reported having significantly more children compared with those without BPD (b = 0.06; SE, 0.01; t = 4.09; P < .001; the b coefficient corresponding to a 6% difference between the 2 groups). Number of marriages and percentage of White participants did not significantly differ between the 2 groups. Model fit indices all indicated an excellent fit (comparative fit index, 0.990; Tucker-Lewis index, 0.973; root mean square error of approximation, 0.019 [95% CI, 0.017-0.021]; standardized root mean square residual, 0.030). The weighted percentages of categorical items and mean values of continuous items are provided in eTable 4 in Supplement 1. The observed correlation matrix can be found in eTable 5 in Supplement 1.
After adjusting for sex, age, and race, the model’s results indicated that (1) the early life adversity score was associated with the reproduction/maintenance trade-off latent factor score (standardized b = 0.448; SE, 0.010; P < .001); (2) the reproduction/maintenance trade-off latent factor score was positively associated with the occurrence of BPD diagnosis (standardized b = 0.335; SE, 0.032; P < .001), and so was (3) the early life adversity score (standardized b = 0.136; SE, 0.019; P < .001) (Figure 1 and eFigure 2 in Supplement 1).
Figure 1. Main Latent Mediation Model.
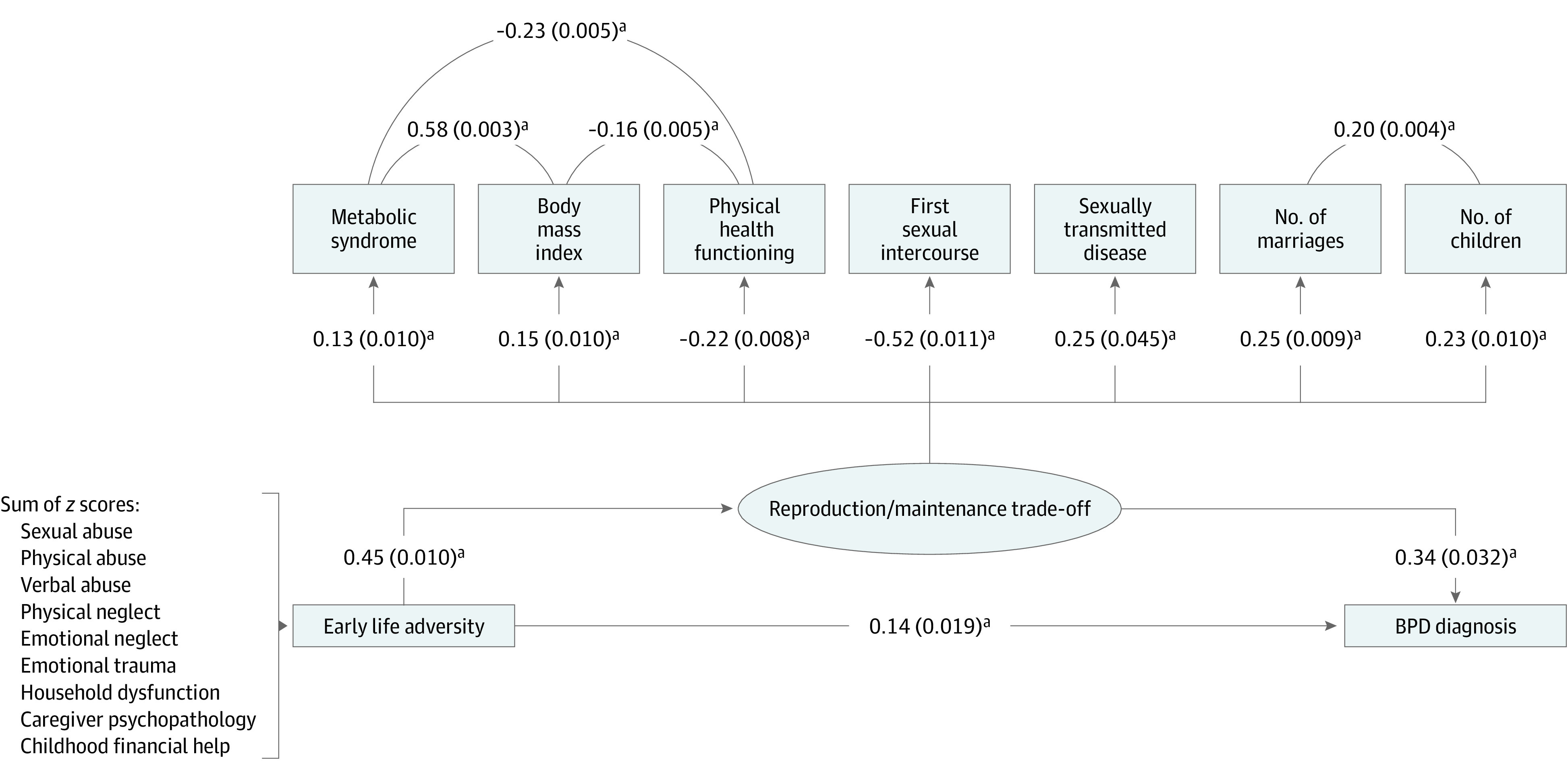
This diagram describes the parameters of the model estimating the direct and indirect associations of early life adversity with the presence or absence of a borderline personality disorder (BPD) diagnosis. The ellipse represents the latent variable; rectangles represent its indicators. Early life adversity is modeled as a single composite variable, represented here by a rectangle. Paths between early life adversity, the reproduction/maintenance trade-off latent factor, and BPD diagnosis represent regressions. Paths between indicators and the reproduction/maintenance trade-off latent factor represent factor loadings.
aP < .001.
These results indicate mediation of the association between adversity in early life and BPD diagnosis in adulthood by a latent reproductive-maintenance trade-off factor. Indeed, the association between early life adversity and BPD in the latent mediation model (b = 0.136; SE, 0.019; P < .001; Figure 1) was reduced in magnitude, relative to the same association in a simple probit regression model that did not include the reproduction/maintenance trade-off latent mediator (b = 0.284; SE, 0.011; P < .001).
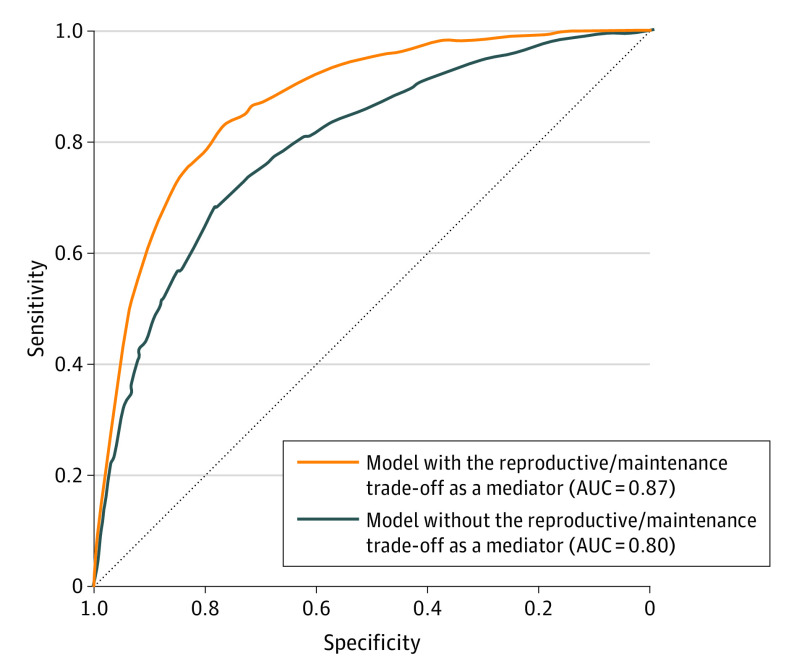
The validity of the latent mediator model was further supported by an analysis of its capacity to accurately indicate the presence or absence of BPD diagnosis (eMethods 3 in Supplement 1). The classification performance of this model was indeed higher than the classification performance of a simple probit regression (area under the receiver operating characteristic of the latent mediator model = 0.87; area under the receiver operating characteristic of probit regression = 0.80; Figure 2).
Figure 2. Classification Performance of the Main Latent Mediation Model.
The orange receiver operating characteristic curve represents the classification performance of the latent mediator model. The blue receiver operating characteristic curve represents the classification performance of a simple regression of the BPD borderline personality disorder variable on the early life adversity variable, which did not include the latent mediator of the reproduction/maintenance trade-off. For sensitivity, borderline personality disorder was present when present. For specificity, borderline personality disorder was absent when absent. AUC indicates area under the curve.
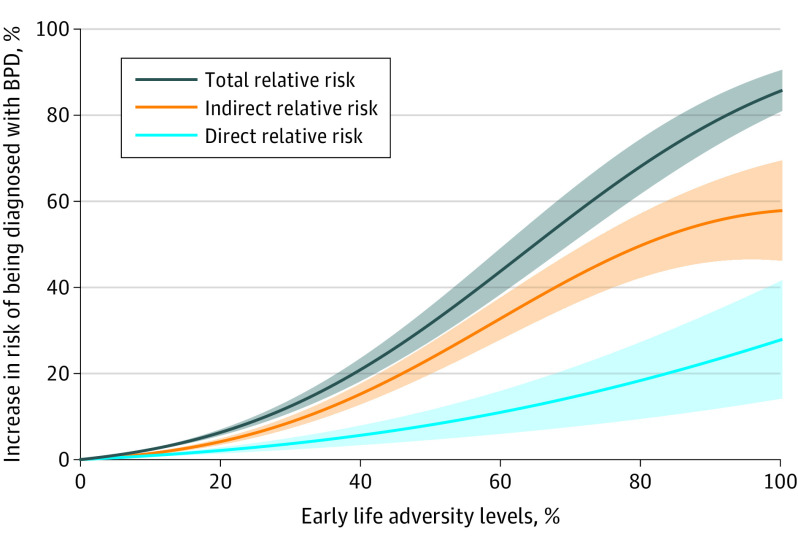
Finally, we conducted an analysis that quantified the extent to which the risk of being diagnosed with BPD in adulthood after experiencing the highest levels of adversity in childhood (eMethods 3 in Supplement 1) could be modulated by individuals’ life strategy.54 This analysis showed that having experienced the highest level of adversity in childhood increased the risk of being diagnosed with BPD by 26.8% (direct relative risk = 0.268; SE, 0.067; P < .001). The latent trade-off factor, indicative of a strategy leading to more effort in immediate reproduction and less effort in somatic maintenance, further increased this risk by 56.5% (indirect relative risk = 0.565; SE, 0.056; P < .001). This value represents 67.8% of the total increase in risk of being diagnosed with BPD after having experienced the highest level of adversity in childhood (Figure 3). The estimates were similar across sex groups, with the exception of minor variations that we detail in eTables 6 and 7 and eFigures 3, 4, and 5 in Supplement 1.
Figure 3. Percent Increase in Risk of Having a Diagnosis of Borderline Personality Disorder (BPD).
Percent increase in risk of having a diagnosis of BPD due to indirect, direct, and total associations between early life adversity and the presence or absence of a BPD diagnosis, for all levels of early life adversity. The orange line and its shaded 95% CI indicates indirect relative risk54 between early life adversity and the presence or absence of a BPD diagnosis through the latent factor of the reproduction/maintenance trade-off, defined as the difference between the probability of having a diagnosis of BPD if one changes the latent factor score in the presence of a lower level of early life adversity, and the latent factor score expected if there had been an increase in early life adversity from its lower to its higher level, while keeping the value of the early life adversity variable fixed (at its upper bound). The light blue line and its shaded 95% CI indicates direct relative risk54 between early life adversity and the presence or absence of a BPD diagnosis, defined as the difference between the probability of having a diagnosis of BPD if the level of early life adversity is changed from its lower to its higher level, while keeping the latent factor score of the reproduction/maintenance trade-off at its expected value in the presence of a lower level of early life adversity. The dark blue line and its shaded 95% CI indicates the total relative risk between early life adversity and the presence or absence of a BPD diagnosis, defined as the sum of the direct and indirect relative risk.54 The median early life adversity level is taken as the baseline level (0).
Sensitivity analyses confirmed that the mediation of the reproduction/maintenance trade-off latent factor remained significant. Notably, it remained significant after adjusting for all lifetime axis I and II disorders (eFigures 6, 7, 8, and 9 in Supplement 1).
The 10-fold cross-validation revealed that the latent mediation model provided both very good fit indices and classification performance across training and test subsamples (eTable 8 and eFigure 10 in Supplement 1).
Discussion
The objectives of this study were to test the existence of a latent factor by which seemingly disparate somatic traits and reproductive behaviors could follow a biologically plausible logic of organization and to examine how this latent factor is associated with early life adversity on the one hand and to the occurrence of BPD at adulthood on the other hand. To our knowledge, no prior theory explains the highly prevalent co-occurrence, in the BPD population (in comparison with population with other disorders, see15,16), of short-term reproductive behaviors and somatic comorbidities. Therefore, we sought to usefully exploit the concept of a trade-off between somatic maintenance and reproduction, predicted by life history theory, to account for the ecological origins of these distinct phenotypic expressions often reported independently in the BPD literature.
The results of our main SEM (Figure 1) support our initial hypothesis, showing that (1) respondents who scored high on the latent trade-off factor, ie, those who pursued immediate reproductive goals and who reported poorer somatic maintenance and health, were more likely to be diagnosed with BPD; (2) experiencing conditions of high adversity is associated with higher scores on the latent trade-off factor and an increased risk of meeting criteria for BPD in adulthood; and (3) the higher the respondents’ score on the latent trade-off factor, the more early life adversity is associated with BPD. An analysis of the latter association further shows that, for respondents who experienced high levels of adversity in early life, scoring high on the latent trade-off factor is associated with increased risk of having a diagnosis of BPD by 56.5% (Figure 3). This pattern of associations was observed in both male and female individuals, with only small differences between the 2 sexes (eMethods 4 and eFigure 5 in Supplement 1). Finally, cross-validation of the main SEM model highlights its ability to generalize its predictions to out-of-sample data, which confirms its robustness (Figure 2).
These results extend those of Otto and colleagues,32 who also examined associations between childhood adversity, somatic traits, and sociosexual preferences in patients with BPD. Our results support a view of BPD as the psychobehavioral expression of a broader coping strategy whereby individuals compensate for the adaptive costs of adverse life conditions by prioritizing a phenotype that provides rapid reproductive benefits at the expense of longer-term health and survival. According to this view, core components of borderline personality, eg, impulsivity, risk-taking, or negative emotionality, function to facilitate earlier sexuality, sexual promiscuity, and intrasexual competition for status and partners. Depressive symptoms and suicidal behaviors may also contribute to the construction of social support networks by eliciting empathy from others, thereby increasing the individual’s value as a socially desirable partner.55
The idea that BPD confers an early advantage in terms of reproductive success seems challenged by the statistics comparing the mean number of children of the BPD and non-BPD subsamples; in the former, individuals reported on average fewer children than in the latter (Table). However, this reproductive difference is only apparent and is explained by the 7.4-year difference in the mean age of the 2 groups, with the mean age of individuals with BPD of 40.5 years compared with 48 years for those without BPD. This large age difference biases the length of the reproductive window against the BPD sample (particularly for males who are not subject to menopause) and therefore likely inflated the mean fertility age of the sample without BPD. Indeed, when age is adjusted for in a log-linear regression with number of children as the dependent variable, subsample (BPD vs non-BPD) as the predictor, and age of respondent as the covariate, individuals with BPD reported having significantly more children than those without BPD. Note that all our SEM models are adjusted for age.
Our results neither invalidate nor minimize the value of alternative models emphasizing the influence of other proximal mechanisms preceding BPD expression.56,57,58,59 For example, Linehan's theory assumes that BPD is a consequence of emotional dysregulation.60 Gunderson and colleagues57 suggest that BPD is a consequence of feelings of loneliness and threat of rejection. Overall, the impact of emotional regulation mechanisms on symptom expression and their sociosexual correlates is a particularly important object of study insofar as these mechanisms constitute accessible therapeutic targets. Future work should integrate the mechanisms uncovered in this study with prior theories. While some recent theoretical contributions already point in this direction,55,61,62 their empirical validation remains to be conducted. Our work assumes that the emergence of BPD is a developmental response to adverse early life experiences. Consistent with this view, the heritability of BPD, estimated at 46% in a recent study,63 is lower than the heritability of attention-deficit/hyperactivity disorder, anorexia nervosa, autism spectrum disorder, bipolar disorder, or schizophrenia.64 Furthermore, heritability models assume that gene and environment have additive effects on the phenotype and thus necessarily underestimate the contribution of gene-environment interactions.65 Life history approaches to personality disorders integrate genetic causation and suggest that early life gene-environment interactions21 may increase the likelihood of expressing a reproduction-oriented life strategy, thereby increasing the risk of BPD.61
Limitations
These findings must be balanced with several limitations. A first limitation is that important proxies of reproductive maturity (eg, age at puberty), which are thought to index reproduction-oriented life strategies, are not available in the NESARC. Second, information regarding institutionalized (incarcerated or hospitalized) or younger individuals is missing from NESARC,42 and future studies will need to determine whether our results generalize to these vulnerable populations. Third, axis I and II disorders were assessed by lay interviewers rather than mental health professionals, which might have resulted in false positives or negatives.16 However, the similarities between the NESARC results (using the same BPD definition we used in this study) and other BPD studies support the validity of the NESARC BPD assessment.2,42,43 Fourth, our indicators were retrospective and/or self-reporting in nature, hence subject to social desirability biases.66 For instance, adults tend to underreport experiences of early adversity.67,68 However, concerns about systematic biases in retrospective reports are mitigated by other prospective studies.69,70 Finally, our use of a cumulative model of adversity is questionable. Indeed, a recent trend in developmental psychopathology research emphasizes the predictive value of dimensional models.71,72
Despite its limitations, our study joins a growing body of work promoting a change in the functional status classically attributed to BDP.25,30,31,32,61 Whereas the common model of medical research views BPD and its multiple physiological and behavioral correlates as dysfunctions, evolutionarily informed approaches suggest that BPD-related traits offer advantages, not in terms of health but in terms of biological fitness, for navigating adverse environments where the risks of dying young or living in poor health conditions are higher.21,30,73
Conclusions
Together, this work opens up promising avenues for improving the prevention and the management of this disorder. First, it highlights the importance of taking somatic and reproductive traits into account in clinical management. In practical terms, informing patients with BPD about the consequences of short-term reproductive behaviors could prevent them from falling into many psychosocial pitfalls, eg, unwanted parenthood, unstable interpersonal relationships, and sexual trauma. Furthermore, if an investment in immediate reproduction leads to less investment in somatic maintenance and thus to health problems later in life,74 it is all the more important to seek reducing reproductive impulsivity. These observations underscore the importance of coordinating mental health services with reproductive and general health services.
eMethods 1. List of items and scoring methods of the variables included in the models
eMethods 2. Confirmatory factor analysis (CFA)
eMethods 3. Structural equation model (SEM)
eMethods 4. Model and estimates variations across sex
eMethods 5. Sensitivity analysis
eMethods 6. 10-Fold cross validation
eTable 1. Items included in the structural equation models (RS = Reverse Coding)
eTable 2. Univariate higher-order moment descriptive statistics
eTable 3. Confirmatory Factor Analysis: correlation matrix of the variables included
eTable 4. Univariate higher-order moment descriptive statistics
eTable 5. Latent mediator model: correlation matrix of the variables included
eTable 6. Descriptive statistics: men vs. women, whole sample
eTable 7. Descriptive statistics: men vs. women, BPD sample
eTable 8. 10-Fold cross validation
eFigure 1. Confirmatory Factor Analysis (CFA) of the reproduction/maintenance trade-off latent construct
eFigure 2. Latent mediator model classification performance versus simple probit regression classification performance
eFigure 3. Latent mediator model in the women sample
eFigure 4. Latent mediator model in the men sample
eFigure 5. Partial metric invariance between men and women
eFigure 6. Exclusion of all respondents who have been forced to have sexual intercourse during their infancy
eFigure 7. Inclusion of an additional adjustment for participants’ personal income in the previous year
eFigure 8. Inclusion of an additional adjustment for all lifetime Axis I and Axis II disorders
eFigure 9. Age limit below 14 years old for childhood adverse events
eFigure 10. 10-Fold cross validation: Area Under the Curve (AUC) of the Receiver Operating Characteristic (ROC) curves across train and test subsamples
eReferences
Data sharing statement
References
- 1.Martín-Blanco A, Ancochea A, Soler J, Elices M, Carmona C, Pascual JC. Changes over the last 15 years in the psychopharmacological management of persons with borderline personality disorder. Acta Psychiatr Scand. 2017;136(3):323-331. doi: 10.1111/acps.12767 [DOI] [PubMed] [Google Scholar]
- 2.Gunderson JG, Herpertz SC, Skodol AE, Torgersen S, Zanarini MC. Borderline personality disorder. Nat Rev Dis Primers. 2018;4:18029. doi: 10.1038/nrdp.2018.29 [DOI] [PubMed] [Google Scholar]
- 3.Chen EY, Brown MZ, Lo TTY, Linehan MM. Sexually transmitted disease rates and high-risk sexual behaviors in borderline personality disorder versus borderline personality disorder with substance use disorder. J Nerv Ment Dis. 2007;195(2):125-129. doi: 10.1097/01.nmd.0000254745.35582.f6 [DOI] [PubMed] [Google Scholar]
- 4.Kaufman EA, Perez J, Lazarus S, Stepp SD, Pedersen SL. Understanding the association between borderline personality disorder and alcohol-related problems: An examination of drinking motives, impulsivity, and affective instability. Personal Disord. 2020;11(3):213-221. doi: 10.1037/per0000375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sansone RA, Barnes J, Muennich E, Wiederman MW. Borderline personality symptomatology and sexual impulsivity. Int J Psychiatry Med. 2008;38(1):53-60. doi: 10.2190/PM.38.1.e [DOI] [PubMed] [Google Scholar]
- 6.Sansone RA, Lam C, Wiederman MW. The relationship between borderline personality disorder and number of sexual partners. J Pers Disord. 2011;25(6):782-788. doi: 10.1521/pedi.2011.25.6.782 [DOI] [PubMed] [Google Scholar]
- 7.Brüne MOJ, Schojai M, Decker C, Edel MA. Mating strategies and experience of early adversity in female patients with borderline personality disorder: insights from life history theory. Pers Individ Dif. 2017;113:147-154. doi: 10.1016/j.paid.2017.03.024 [DOI] [Google Scholar]
- 8.King-Casas B, Sharp C, Lomax-Bream L, Lohrenz T, Fonagy P, Montague PR. The rupture and repair of cooperation in borderline personality disorder. Science. 2008;321(5890):806-810. doi: 10.1126/science.1156902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ebert A, Kolb M, Heller J, Edel MA, Roser P, Brüne M. Modulation of interpersonal trust in borderline personality disorder by intranasal oxytocin and childhood trauma. Soc Neurosci. 2013;8(4):305-313. doi: 10.1080/17470919.2013.807301 [DOI] [PubMed] [Google Scholar]
- 10.Shackelford T, Goetz A, Buss D, Euler H, Hoier S. When we hurt the ones we love: predicting violence against women from men’s mate retention. Pers Relatsh. 2005;12:447-463. doi: 10.1111/j.1475-6811.2005.00125.x [DOI] [Google Scholar]
- 11.Zanarini MC, Frankenburg FR, Reich DB, Fitzmaurice G, Weinberg I, Gunderson JG. The 10-year course of physically self-destructive acts reported by borderline patients and axis II comparison subjects. Acta Psychiatr Scand. 2008;117(3):177-184. doi: 10.1111/j.1600-0447.2008.01155.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morgan TA, Chelminski I, Young D, Dalrymple K, Zimmerman M. Differences between older and younger adults with borderline personality disorder on clinical presentation and impairment. J Psychiatr Res. 2013;47(10):1507-1513. doi: 10.1016/j.jpsychires.2013.06.009 [DOI] [PubMed] [Google Scholar]
- 13.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013. [Google Scholar]
- 14.Aouidad A, Cohen D, Mirkovic B, et al. Borderline personality disorder and prior suicide attempts define a severity gradient among hospitalized adolescent suicide attempters. BMC Psychiatry. 2020;20(1):525. doi: 10.1186/s12888-020-02930-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moran P, Stewart R, Brugha T, et al. Personality disorder and cardiovascular disease: results from a national household survey. J Clin Psychiatry. 2007;68(1):69-74. doi: 10.4088/JCP.v68n0109 [DOI] [PubMed] [Google Scholar]
- 16.El-Gabalawy R, Katz LY, Sareen J. Comorbidity and associated severity of borderline personality disorder and physical health conditions in a nationally representative sample. Psychosom Med. 2010;72(7):641-647. doi: 10.1097/PSY.0b013e3181e10c7b [DOI] [PubMed] [Google Scholar]
- 17.Stepp SD, Lazarus SA, Byrd AL. A systematic review of risk factors prospectively associated with borderline personality disorder: taking stock and moving forward. Personal Disord. 2016;7(4):316-323. doi: 10.1037/per0000186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hughes K, Bellis MA, Hardcastle KA, et al. The effect of multiple adverse childhood experiences on health: a systematic review and meta-analysis. Lancet Public Health. 2017;2(8):e356-e366. doi: 10.1016/S2468-2667(17)30118-4 [DOI] [PubMed] [Google Scholar]
- 19.Porter C, Palmier-Claus J, Branitsky A, Mansell W, Warwick H, Varese F. Childhood adversity and borderline personality disorder: a meta-analysis. Acta Psychiatr Scand. 2020;141(1):6-20. doi: 10.1111/acps.13118 [DOI] [PubMed] [Google Scholar]
- 20.West-Eberhard MJ. Developmental Plasticity and Evolution. Oxford University Press; 2003. doi: 10.1093/oso/9780195122343.001.0001 [DOI] [Google Scholar]
- 21.Ellis BJ, Del Giudice M. Developmental adaptation to stress: an evolutionary perspective. Annu Rev Psychol. 2019;70:111-139. doi: 10.1146/annurev-psych-122216-011732 [DOI] [PubMed] [Google Scholar]
- 22.Kenrick DT, Griskevicius V, Neuberg SL, Schaller M. Renovating the pyramid of needs: contemporary extensions built upon ancient foundations. Perspect Psychol Sci. 2010;5(3):292-314. doi: 10.1177/1745691610369469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stearns SC. The Evolution of Life Histories. OUP Oxford; 1992. [Google Scholar]
- 24.Muehlenbein MP, Flinn MV. Patterns and processes of human life history evolution. In: Flatt T, Heyland A, eds. Mechanisms of Life History Evolution. Oxford University Press; 2011:153-168. doi: 10.1093/acprof:oso/9780199568765.003.0012 [DOI] [Google Scholar]
- 25.Brüne M, Ghiassi V, Ribbert H. Does borderline personality disorder reflect the pathological extreme of an adaptive reproductive strategy? insights and hypotheses from evolutionary life-history theory. Clinical Neuropsychiatry. 2010;7(1):3-9. [Google Scholar]
- 26.Harned MS, Pantalone DW, Ward-Ciesielski EF, Lynch TR, Linehan MM. The prevalence and correlates of sexual risk behaviors and sexually transmitted infections in outpatients with borderline personality disorder. J Nerv Ment Dis. 2011;199(11):832-838. doi: 10.1097/NMD.0b013e318234c02c [DOI] [PubMed] [Google Scholar]
- 27.De Genna NM, Feske U, Larkby C, Angiolieri T, Gold MA. Pregnancies, abortions, and births among women with and without borderline personality disorder. Womens Health Issues. 2012;22(4):e371-e377. doi: 10.1016/j.whi.2012.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.MacDowell KS, Marsá MD, Buenache E, et al. Inflammatory and antioxidant pathway dysfunction in borderline personality disorder. Psychiatry Res. 2020;284:112782. doi: 10.1016/j.psychres.2020.112782 [DOI] [PubMed] [Google Scholar]
- 29.Saccaro LF, Schilliger Z, Dayer A, Perroud N, Piguet C. Inflammation, anxiety, and stress in bipolar disorder and borderline personality disorder: a narrative review. Neurosci Biobehav Rev. 2021;127:184-192. doi: 10.1016/j.neubiorev.2021.04.017 [DOI] [PubMed] [Google Scholar]
- 30.Brüne M. Borderline personality disorder: why ‘fast and furious’? Evol Med Public Health. 2016;2016(1):52-66. doi: 10.1093/emph/eow002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baptista A, Cohen D, Jacquet PO, Chambon V. The cognitive, ecological, and developmental origins of self-disturbance in borderline personality disorder. Front Psychiatry. 2021;12:707091. doi: 10.3389/fpsyt.2021.707091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Otto B, Kokkelink L, Brüne M. Borderline personality disorder in a “life history theory” perspective: evidence for a fast “pace-of-life-syndrome”. Front Psychol. 2021;12:715153. doi: 10.3389/fpsyg.2021.715153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573-577. doi: 10.7326/0003-4819-147-8-200710160-00010 [DOI] [PubMed] [Google Scholar]
- 34.Grant BF, Stinson FS, Dawson DA, et al. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2004;61(8):807-816. doi: 10.1001/archpsyc.61.8.807 [DOI] [PubMed] [Google Scholar]
- 35.Grant BF, Goldstein RB, Chou SP, et al. Sociodemographic and psychopathologic predictors of first incidence of DSM-IV substance use, mood and anxiety disorders: results from the Wave 2 National Epidemiologic Survey on Alcohol and Related Conditions. Mol Psychiatry. 2009;14(11):1051-1066. doi: 10.1038/mp.2008.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blanco C, Wall MM, Hoertel N, et al. Psychiatric disorders and risk for multiple adverse outcomes: a national prospective study. Mol Psychiatry. 2021;26(3):907-916. doi: 10.1038/s41380-019-0459-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brumbach BH, Figueredo AJ, Ellis BJ. Effects of harsh and unpredictable environments in adolescence on development of life history strategies: a longitudinal test of an evolutionary model. Hum Nat. 2009;20(1):25-51. doi: 10.1007/s12110-009-9059-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mell H, Safra L, Algan Y, Baumard N, Chevallier C. Childhood environmental harshness predicts coordinated health and reproductive strategies: a cross-sectional study of a nationally representative sample from France. Evol Hum Behav. 2018;39(1):1-8. doi: 10.1016/j.evolhumbehav.2017.08.006 [DOI] [Google Scholar]
- 39.Ruan WJ, Goldstein RB, Chou SP, et al. The alcohol use disorder and associated disabilities interview schedule-IV (AUDADIS-IV): reliability of new psychiatric diagnostic modules and risk factors in a general population sample. Drug Alcohol Depend. 2008;92(1-3):27-36. doi: 10.1016/j.drugalcdep.2007.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th ed. American Psychiatric Association; 1994. [Google Scholar]
- 41.Trull TJ, Jahng S, Tomko RL, Wood PK, Sher KJ. Revised NESARC personality disorder diagnoses: gender, prevalence, and comorbidity with substance dependence disorders. J Pers Disord. 2010;24(4):412-426. doi: 10.1521/pedi.2010.24.4.412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hoertel N, Peyre H, Wall MM, Limosin F, Blanco C. Examining sex differences in DSM-IV borderline personality disorder symptom expression using Item Response Theory (IRT). J Psychiatr Res. 2014;59:213-219. doi: 10.1016/j.jpsychires.2014.08.019 [DOI] [PubMed] [Google Scholar]
- 43.McMahon K, Hoertel N, Peyre H, Blanco C, Fang C, Limosin F. Age differences in DSM-IV borderline personality disorder symptom expression: results from a national study using item response theory (IRT). J Psychiatr Res. 2019;110:16-23. doi: 10.1016/j.jpsychires.2018.12.019 [DOI] [PubMed] [Google Scholar]
- 44.Nettle D, Coall DA, Dickins TE. Birthweight and paternal involvement predict early reproduction in British women: evidence from the National Child Development Study. Am J Hum Biol. 2010;22(2):172-179. [DOI] [PubMed] [Google Scholar]
- 45.Simpson JA, Griskevicius V, Kuo SIC, Sung S, Collins WA. Evolution, stress, and sensitive periods: the influence of unpredictability in early versus late childhood on sex and risky behavior. Dev Psychol. 2012;48(3):674-686. doi: 10.1037/a0027293 [DOI] [PubMed] [Google Scholar]
- 46.Pepper GV, Nettle D. Out of control mortality matters: the effect of perceived uncontrollable mortality risk on a health-related decision. PeerJ. 2014;2:e459. doi: 10.7717/peerj.459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lettinga N, Mell H, Algan Y, Jacquet PO, Chevallier C. Childhood environmental adversity is not linked to lower levels of cooperative behaviour in economic games. Evol Hum Sci. 2021;3:e29. doi: 10.1017/ehs.2021.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lumley T. Complex Surveys: A Guide to Analysis Using R. Vol 565. John Wiley & Sons; 2011. [Google Scholar]
- 49.Muthén LK, Muthén B. Mplus User’s Guide: Statistical Analysis with Latent Variables, User’s Guide. Muthén & Muthén; 2017. [Google Scholar]
- 50.Asparouhov T, Muthén B. Simple second order chi-square correction. Accessed March 15, 2023. https://www.statmodel.com/download/WLSMV_new_chi21.pdf
- 51.Breckler SJ. Applications of covariance structure modeling in psychology: cause for concern? Psychol Bull. 1990;107(2):260-273. doi: 10.1037/0033-2909.107.2.260 [DOI] [PubMed] [Google Scholar]
- 52.Roberts S, Pashler H. How persuasive is a good fit? a comment on theory testing. Psychol Rev. 2000;107(2):358-367. doi: 10.1037/0033-295X.107.2.358 [DOI] [PubMed] [Google Scholar]
- 53.Preacher KJ. Quantifying parsimony in structural equation modeling. Multivariate Behav Res. 2006;41(3):227-259. doi: 10.1207/s15327906mbr4103_1 [DOI] [PubMed] [Google Scholar]
- 54.Muthén B, Asparouhov T. Causal effects in mediation modeling: an introduction with applications to latent variables. Struct Equ Modeling. 2015;22(1):12-23. doi: 10.1080/10705511.2014.935843 [DOI] [Google Scholar]
- 55.Martel MM. Sexual selection and sex differences in the prevalence of childhood externalizing and adolescent internalizing disorders. Psychol Bull. 2013;139(6):1221-1259. doi: 10.1037/a0032247 [DOI] [PubMed] [Google Scholar]
- 56.Kernberg OF. Borderline Conditions and Pathological Narcissism. Rowman & Littlefield; 1985. [Google Scholar]
- 57.Gunderson JG, Lyons-Ruth K. BPD’s interpersonal hypersensitivity phenotype: a gene-environment-developmental model. J Pers Disord. 2008;22(1):22-41. doi: 10.1521/pedi.2008.22.1.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bateman A, Fonagy P. Borderline personality disorder and mood disorders: mentalizing as a framework for integrated treatment. J Clin Psychol. 2015;71(8):792-804. doi: 10.1002/jclp.22206 [DOI] [PubMed] [Google Scholar]
- 59.Clarkin JF, Caligor E, Sowislo JF. An object relations model perspective on the alternative model for personality disorders (DSM-5). Psychopathology. 2020;53(3-4):141-148. doi: 10.1159/000508353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Crowell SE, Beauchaine TP, Linehan MM. A biosocial developmental model of borderline personality: elaborating and extending Linehan’s theory. Psychol Bull. 2009;135(3):495-510. doi: 10.1037/a0015616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Del Giudice M, Haltigan JD. An integrative evolutionary framework for psychopathology. Dev Psychopathol. 2023;35(1):1-11. doi: 10.1017/S0954579421000870 [DOI] [PubMed] [Google Scholar]
- 62.Del Giudice M. A general motivational architecture for human and animal personality. Neurosci Biobehav Rev. 2023;144:104967. doi: 10.1016/j.neubiorev.2022.104967 [DOI] [PubMed] [Google Scholar]
- 63.Skoglund C, Tiger A, Rück C, et al. Familial risk and heritability of diagnosed borderline personality disorder: a register study of the Swedish population. Mol Psychiatry. 2021;26(3):999-1008. doi: 10.1038/s41380-019-0442-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Baselmans BML, Yengo L, van Rheenen W, Wray NR. Risk in relatives, heritability, SNP-based heritability, and genetic correlations in psychiatric disorders: a review. Biol Psychiatry. 2021;89(1):11-19. doi: 10.1016/j.biopsych.2020.05.034 [DOI] [PubMed] [Google Scholar]
- 65.Visscher PM, Hill WG, Wray NR. Heritability in the genomics era: concepts and misconceptions. Nat Rev Genet. 2008;9(4):255-266. doi: 10.1038/nrg2322 [DOI] [PubMed] [Google Scholar]
- 66.Krumpal I. Determinants of social desirability bias in sensitive surveys: a literature review. Qual Quant. 2013;47(4):2025-2047. doi: 10.1007/s11135-011-9640-9 [DOI] [Google Scholar]
- 67.Keyes KM, Eaton NR, Krueger RF, et al. Childhood maltreatment and the structure of common psychiatric disorders. Br J Psychiatry. 2012;200(2):107-115. doi: 10.1192/bjp.bp.111.093062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brown MJ, Perera RA, Masho SW, Mezuk B, Cohen SA. Adverse childhood experiences and intimate partner aggression in the US: sex differences and similarities in psychosocial mediation. Soc Sci Med. 2015;131:48-57. doi: 10.1016/j.socscimed.2015.02.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fergusson DM, Horwood LJ, Woodward LJ. The stability of child abuse reports: a longitudinal study of the reporting behaviour of young adults. Psychol Med. 2000;30(3):529-544. doi: 10.1017/S0033291799002111 [DOI] [PubMed] [Google Scholar]
- 70.Scott KM, Smith DR, Ellis PM. Prospectively ascertained child maltreatment and its association with DSM-IV mental disorders in young adults. Arch Gen Psychiatry. 2010;67(7):712-719. doi: 10.1001/archgenpsychiatry.2010.71 [DOI] [PubMed] [Google Scholar]
- 71.Farkas BC, Baptista A, Speranza M, Wyart V, Jacquet PO. Specifying the timescale of early life unpredictability helps explain individual differences in children’s internalising and externalising symptoms. PsyArXiv. Preprint posted March 22, 2022. doi: 10.31234/osf.io/t5avy [DOI]
- 72.McLaughlin KA, Sheridan MA, Humphreys KL, Belsky J, Ellis BJ. The value of dimensional models of early experience: thinking clearly about concepts and categories. Perspect Psychol Sci. 2021;16(6):1463-1472. doi: 10.1177/1745691621992346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ellis BJ, Abrams LS, Masten AS, Sternberg RJ, Tottenham N, Frankenhuis WE. Hidden talents in harsh environments. Dev Psychopathol. 2022;34(1):95-113. doi: 10.1017/S0954579420000887 [DOI] [PubMed] [Google Scholar]
- 74.Jasienska G, Bribiescas RG, Furberg AS, Helle S, Núñez-de la Mora A. Human reproduction and health: an evolutionary perspective. Lancet. 2017;390(10093):510-520. doi: 10.1016/S0140-6736(17)30573-1 [DOI] [PubMed] [Google Scholar]
- 75.Taylor J, Turner RJ. Perceived discrimination, social stress, and depression in the transition to adulthood: racial contrasts. Soc Psychol Quarterly. 2002;65(3):213-225. [Google Scholar]
- 76.Pascoe EA, Smart Richman L. Perceived discrimination and health: a meta-analytic review. Psychol Bull. 2009;135(4):531-554. doi: 10.1037/a0016059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Williams DR, Lawrence JA, Davis BA. Racism and health: evidence and needed research. Annu Rev Public Health. 2019;40:105-125. doi: 10.1146/annurev-publhealth-040218-043750 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods 1. List of items and scoring methods of the variables included in the models
eMethods 2. Confirmatory factor analysis (CFA)
eMethods 3. Structural equation model (SEM)
eMethods 4. Model and estimates variations across sex
eMethods 5. Sensitivity analysis
eMethods 6. 10-Fold cross validation
eTable 1. Items included in the structural equation models (RS = Reverse Coding)
eTable 2. Univariate higher-order moment descriptive statistics
eTable 3. Confirmatory Factor Analysis: correlation matrix of the variables included
eTable 4. Univariate higher-order moment descriptive statistics
eTable 5. Latent mediator model: correlation matrix of the variables included
eTable 6. Descriptive statistics: men vs. women, whole sample
eTable 7. Descriptive statistics: men vs. women, BPD sample
eTable 8. 10-Fold cross validation
eFigure 1. Confirmatory Factor Analysis (CFA) of the reproduction/maintenance trade-off latent construct
eFigure 2. Latent mediator model classification performance versus simple probit regression classification performance
eFigure 3. Latent mediator model in the women sample
eFigure 4. Latent mediator model in the men sample
eFigure 5. Partial metric invariance between men and women
eFigure 6. Exclusion of all respondents who have been forced to have sexual intercourse during their infancy
eFigure 7. Inclusion of an additional adjustment for participants’ personal income in the previous year
eFigure 8. Inclusion of an additional adjustment for all lifetime Axis I and Axis II disorders
eFigure 9. Age limit below 14 years old for childhood adverse events
eFigure 10. 10-Fold cross validation: Area Under the Curve (AUC) of the Receiver Operating Characteristic (ROC) curves across train and test subsamples
eReferences
Data sharing statement