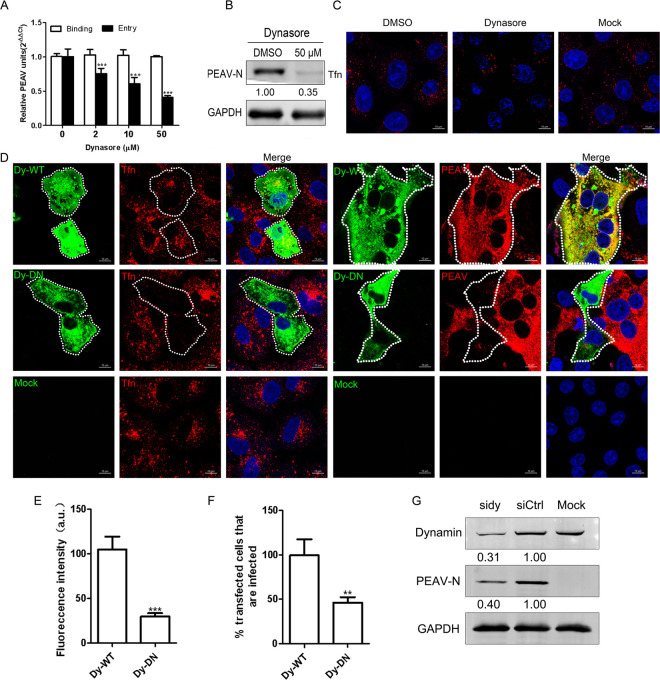
FIG 2.
PEAV entry requires a dynamin. (A) Vero cells were pretreated with dynasore (15 min, 37°C), followed by PEAV (MOI = 1) inoculation to allow binding (1 h, 4°C) and entry (1 h, 37°C) in the presence of drugs. (B) In the presence of dynasore (50 μM), cells were inoculated with PEAV (MOI = 5) (2.5 h, 37°C), followed by Western blotting to detect the early expression of PEAV N protein. Relative amounts of protein were calculated using ImageJ software. (C) Vero cells were pretreated with dynasore (50 μM) or dimethyl sulfoxide (DMSO) (negative control) (15 min, 37°C), followed by incubation with 20 μg/mL Alexa Fluor 568-labeled Tfn (15 min, 37°C), and Tfn uptake was examined using confocal microscopy. (D) Vero cells were transfected with dynamin (WT or DN) plasmid (24 h, 37°C) and then incubated with 20 μg/mL Alexa Fluor 568-labeled Tfn (30 min, 37°C). Cells were fixed (4% PFA, 15 min, room temperature) or inoculated with PEAV (MOI = 5) (6 h, 37°C) and fixed and then immunodetected with anti-PEAV N monoclonal antibody. (E) Total fluorescence intensity per cell was calculated in the cells transfected with dynamin DN plasmid. (F) At least 300 transfected cells were scored positive (+) or negative (−) for PEAV infection; values are expressed as a percentage of infected cells observed in the control sample. (G) Cells were transfected with siDynamin or siCtrl (48 h, 37°C) and inoculated with PEAV (MOI = 5). Viral N protein expression was quantified using Western blotting at 2.5 h postinfection (hpi). The relative protein content was calculated using ImageJ software. All results are presented as the mean ± SD from three independent experiments (*, P < 0.05; **, P < 0.01; ***, P < 0.001). Scale bar = 10 μm.