ABSTRACT
Many RING domain E3 ubiquitin ligases play critical roles in fine-tuning the innate immune response, yet little is known about their regulatory role in flavivirus-induced innate immunity. In previous studies, we found that the suppressor of cytokine signaling 1 (SOCS1) protein mainly undergoes lysine 48 (K48)-linked ubiquitination. However, the E3 ubiquitin ligase that promotes the K48-linked ubiquitination of SOCS1 is unknown. In the present study, we found that RING finger protein 123 (RNF123) binds to the SH2 domain of SOCS1 through its RING domain and facilitates the K48-linked ubiquitination of the K114 and K137 residues of SOCS1. Further studies found that RNF123 promoted the proteasomal degradation of SOCS1 and promoted Toll-like receptor 3 (TLR3)- and interferon (IFN) regulatory factor 7 (IRF7)-mediated type I IFN production during duck Tembusu virus (DTMUV) infection through SOCS1, ultimately inhibiting DTMUV replication. Overall, these findings demonstrate a novel mechanism by which RNF123 regulates type I IFN signaling during DTMUV infection by targeting SOCS1 degradation.
IMPORTANCE In recent years, posttranslational modification (PTM) has gradually become a research hot spot in the field of innate immunity regulation, and ubiquitination is one of the critical PTMs. DTMUV has seriously endangered the development of the waterfowl industry in Southeast Asian countries since its outbreak in 2009. Previous studies have shown that SOCS1 is modified by K48-linked ubiquitination during DTMUV infection, but E3 ubiquitin ligase catalyzing the ubiquitination of SOCS1 has not been reported. Here, we identify for the first time that RNF123 acts as an E3 ubiquitin ligase that regulates TLR3- and IRF7-induced type I IFN signaling during DTMUV infection by targeting the K48-linked ubiquitination of the K114 and K137 residues of SOCS1 and the proteasomal degradation of SOCS1.
KEYWORDS: DTMUV, RNF123, SOCS1, type I IFN, ubiquitination
INTRODUCTION
In 2010, a duck infectious disease with an incidence rate of 90% broke out in China, and the pathogen of the disease was duck Tembusu virus (DTMUV) (1). DTMUV belongs to the genus Flavivirus of the family Flaviviridae, and its infection causes mainly damage to the immune organs, neurological dysfunction, slow growth, and decreased egg production of infected waterfowl, which brought substantial economic losses to the waterfowl industry in China (1–4). Previous studies have found that mammalian cells (such as A549, BHK21, HeLa, SH-SY5Y, and Vero cells) are susceptible to DTMUV infection (5–7). A recent study showed that Kunming mice and BALB/c mice were also sensitive to DTMUV after inoculation with DTMUV in the brain (6, 8). In addition, anti-DTMUV antibodies (Abs) were detected in serum samples of more than 70% of duck farm workers (9). Therefore, these findings suggest that DTMUV may expand its host range and potentially endanger human health.
Innate immunity plays a role in the first line of defense in the process of pathogens invading the body. The specific process is that pathogen recognition receptors (PRRs) activate downstream signaling cascades upon the recognition of pathogen-associated molecular patterns (PAMPs), ultimately stimulating the production of interferons (IFNs) (10, 11). The Toll-like receptor 3 (TLR3)/IFN regulatory factor 7 (IRF7)-mediated innate immunity pathway was found to be activated by DTMUV infection, thus activating type I IFN production (12, 13). Further studies found that the activation of the TLR3 signaling pathway promoted suppressor of cytokine signaling 1 (SOCS1) expression during DTMUV infection but upregulated SOCS1-antagonized type I IFN signaling and promoted DTMUV replication by degrading IRF7 (14). SOCS1 is a crucial negative regulator of cytokines, and many viruses have evolved immune evasion mechanisms that utilize SOCS1 to inhibit type I IFN production. For example, the upregulation of SOCS1 expression during porcine reproductive and respiratory syndrome virus infection suppresses the production of type I IFN and IFN-stimulated genes (ISGs) to promote self-proliferation (15). Human T cell leukemia virus type 1 (HTLV-1) also stimulates the upregulation of SOCS1, which inhibits IRF3-mediated type I IFN signaling and facilitates HTLV-1 proliferation by catalyzing the ubiquitination and degradation of IRF3 (16). Furthermore, influenza A virus (IAV) infection facilitates the production of SOCS1 and SOCS3 to antagonize JAK/STAT signaling and ultimately inhibit type I and II IFN production (17). Previous reports suggested that SOCS1 is modified by lysine 48 (K48)-linked ubiquitination, but which lysine residues of SOCS1 are ubiquitinated and which E3 ubiquitin ligase encourages the ubiquitination of SOCS1 have not been elucidated.
Proteins of the RING finger (RNF) family play an essential role in regulating innate immunity caused by viral infection by acting as E3 ubiquitin ligases. On the one hand, the pseudorabies virus tegument protein UL13 interacts with the cyclic dinucleotide (CDN) domain of stimulator of IFN genes (STING), and on the other hand, UL13 recruits RNF5 to promote the K27/K29-related ubiquitination and proteasomal degradation of STING, thereby inhibiting the STING-mediated expression of type I IFN and downstream antiviral factors (18). Similarly, the Newcastle disease virus V5 protein recruits RNF5 to mediate the ubiquitination of the K362 and K461 sites of MAVS, a key innate immune signaling molecule, leading to the ubiquitination and degradation of MAVS and the blocking of type I IFN production (19). In addition, RNF114 of sea perch is upregulated during red-spotted grouper nervous necrosis virus infection, and upregulated RNF114 targets TRAF3 and MAVS for K27- and K48-related ubiquitination and proteasomal degradation, thereby suppressing type I IFN expression (20). However, the regulatory role of the RNF family in type I IFN signaling during DTMUV infection has not been reported.
Here, we demonstrate that RNF123 binds to the SH2 domain of SOCS1 through its RING domain and promotes the K48-linked ubiquitination of SOCS1 at K114 and K137, resulting in the proteasomal degradation of SOCS1. SOCS1 is a negative regulator of cytokines, and RNF123 promotes TLR3- and IRF7-mediated type I IFN expression by degrading SOCS1. However, RNF123 was significantly downregulated at 60 h of DTMUV infection, so DTMUV may promote the production of SOCS1 by inhibiting the production of RNF123, thereby restraining type I IFN signaling and facilitating its replication.
RESULTS
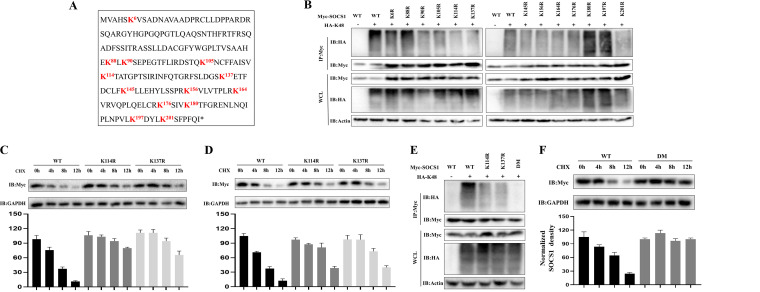
K114 and K137 of SOCS1 are target sites for K48-linked ubiquitination.
As shown in Fig. 1A, there are 13 lysine sites in the duck SOCS1 protein. To determine which lysine site of SOCS1 undergoes K48-linked ubiquitination, we made single point mutations of the 13 lysines to arginine in the sequence. The results revealed that the K48-linked ubiquitination level of SOCS1 was significantly reduced when K114 and K137 of SOCS1 were mutated (Fig. 1B). To further verify the above-mentioned conclusions, we cotransfected hemagglutinin (HA)-tagged K48-linked ubiquitin (HA-Ub-K48) with wild-type (WT) SOCS1 and the K114R and K137R mutants and then treated the cells with the protein synthesis inhibitor cycloheximide (CHX) to determine the half-lives of the wild-type and mutant SOCS1 proteins. It can be seen from Fig. 1C and D that when the K114 and K137 sites of SOCS1 were mutated, the half-life of SOCS1 was significantly prolonged. We further constructed a double-point mutant (DM) in which K114 and K137 were simultaneously mutated and found that the K48-related ubiquitination modification on SOCS1 was almost undetectable when these two lysines were simultaneously mutated (Fig. 1E). Moreover, compared with the WT, the half-life of the DM group was significantly prolonged (Fig. 1F). Therefore, the K114 and K137 sites are the key sites for the K48-linked ubiquitination of SOCS1.
FIG 1.
K114 and K137 of SOCS1 are target sites for K48-linked ubiquitination. (A) Full-length sequence of SOCS1. Lysine residues are in bold red. (B) HEK 293T cells were transfected with HA-Ub-K48 (1.5 mg/well) together with WT or SOCS1 point mutant plasmids (1.5 mg/well). Thirty-six hours later, the cell lysates were immunoprecipitated (IP) with anti-Myc, and ubiquitinated SOCS1 was detected by immunoblotting (IB) with anti-HA. WCL, whole-cell lysate. (C and D) HEK 293T cells or DEFs were transfected with the WT or the K114R or K137R mutant (2.5 mg/well). Thirty-six hours later, the cells were treated with CHX (200 μg/mL). Cells were harvested 0 h, 4 h, 8 h, and 12 h after treatment. The protein level of WT or mutant SOCS1 was determined by IB with anti-Myc. (E) HEK 293T cells were transfected with HA-Ub-K48 (1.5 mg/well) together with the WT, the K114R or K137R single-point mutant, or the DM (1.5 mg/well). Thirty-six hours after transfection, the cell lysates were immunoprecipitated with anti-Myc, and ubiquitinated SOCS1 was detected by IB with anti-HA. (F) HEK 293T cells were transfected with the WT or DM (2.5 mg/well). Thirty-six hours later, the cells were treated with CHX (200 μg/mL). Cells were harvested 0 h, 4 h, 8 h, and 12 h after treatment. The protein level of WT or mutant SOCS1 was determined by IB with anti-Myc. The densitometric values of the SOCS1 protein were quantified and analyzed. All data are the mean values obtained from at least three replicates of independent experiments.
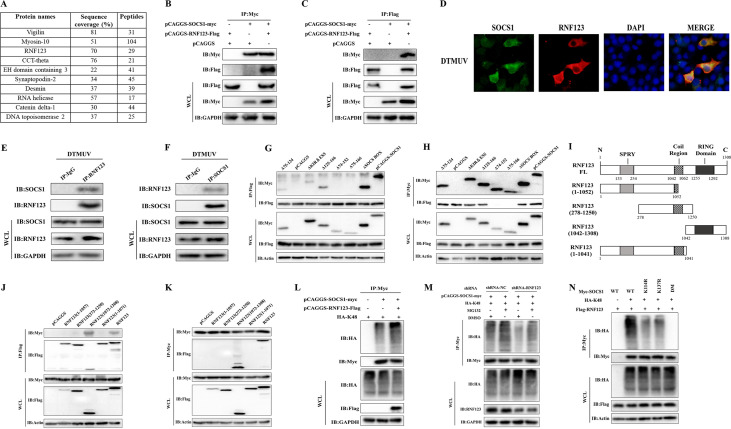
RNF123 binds to the SH2 domain of SOCS1 through its RING domain and promotes the K48-linked ubiquitination of SOCS1.
By mass spectrometry analysis, we found that only one E3 ubiquitin ligase, RNF123, of the top 10 proteins with the highest SOCS1 interaction scores can interact with SOCS1 (Fig. 2A). By coimmunoprecipitation (co-IP) experiments, it was verified that SOCS1 interacted with RNF123 (Fig. 2B and C). The results of indirect immunofluorescence assays showed that SOCS1 and RNF123 were colocated in the cytoplasm during DTMUV infection (Fig. 2D). In addition, endogenous co-IP results showed that endogenous RNF123 could also bind to endogenous SOCS1 directly during DTMUV infection (Fig. 2E and F). Truncations of each domain of SOCS1 were constructed previously (14). Co-IP experiments showed that RNF123 binds mainly to the SH2 domain of SOCS1 (Fig. 2G and H). So, through which domain does RNF123 bind to SOCS1? The software predicted that duck RNF123 has an N-terminal SPRY domain, a central coiled-coil domain, and a C-terminal RING finger domain (Fig. 2I). To test which domain of RNF123 binds to SOCS1, we constructed truncated mutants of RNF123. The results showed that RNF123 binds to SOCS1 through its RING finger domain (Fig. 2J and K). These results also indirectly suggested that RNF123 might mediate the ubiquitination of SOCS1. To test this conjecture, we cotransfected RNF123 with SOCS1 and HA-Ub-K48. The data showed that the cotransfection of RNF123 with SOCS1 significantly upregulated the K48-related ubiquitination level of SOCS1 (Fig. 2L). Furthermore, under the same treatment conditions with dimethyl sulfoxide (DMSO) or the proteasome inhibitor MG132, when SOCS1 was cotransfected with short hairpin RNA targeting RNF123 (shRNA-RNF123), the level of SOCS1 ubiquitination was significantly downregulated compared with that in the shRNA-NC (nontargeting control shRNA) group (Fig. 2M). In the shRNA-NC-transfected group or the shRNA-RNF123-transfected group, treatment with MG132 upregulated the level of SOCS1 ubiquitination compared with that in the DMSO-treated group (Fig. 2M). Thus, RNF123 promotes the K48-related ubiquitination of SOCS1. To further verify the above-mentioned conclusions, we cotransfected RNF123 with the K114R, K137R, DM, and WT proteins and found that the ubiquitination levels of K114R and K137R were significantly lower than those of the WT group (Fig. 2N). When the DM was cotransfected with RNF123, the ubiquitination level of SOCS1 was almost undetectable (Fig. 2N). Collectively, the above-described data demonstrate that the RING finger domain of RNF123 is involved mainly in binding to the SH2 domain of SOCS1 and that RNF123 promotes the K48-related ubiquitination of the K114 and K137 residues of SOCS1.
FIG 2.
RNF123 binds to the SH2 domain of SOCS1 through its RING domain and promotes the K48-linked ubiquitination of SOCS1. (A) Mass spectroscopic analysis of potential SOCS1 binding proteins. The numbers of peptides recognized in interacting proteins (including RNF123) are shown. (B and C) HEK 293T cells were transfected with pCAGGS-RNF123-Flag (1.5 mg/well) and pCAGGS (1.5 mg/well), pCAGGS and pCAGGS-SOCS1-Myc (1.5 mg/well), and pCAGGS-RNF123-Flag and pCAGGS-SOCS1-Myc. Thirty-six hours later, the cell lysates were immunoprecipitated with anti-Myc or anti-Flag, followed by IB with anti-Myc and anti-Flag. (D) When the cell confluence reached about 90%, the cells were infected with DTMUV at an MOI of 1. Thirty-six hours after infection, the cells were collected for indirect immunofluorescence detection of endogenous SOCS1 and RNF123 colocalization (original magnification, ×600). (E and F) DEFs were infected with DTMUV at an MOI of 1. Thirty-six hours later, the cell lysates were immunoprecipitated with anti-SOCS1, anti-RNF123, or IgG, followed by IB with anti-SOCS1 and anti-RNF123. (G and H) HEK 293T cells were transfected with pCAGGS-RNF123-Flag (1.5 mg/well) together with the plasmids for full-length or truncated SOCS1 (1.5 mg/well). Thirty-six hours later, the cell lysates were immunoprecipitated with anti-Myc or anti-Flag, followed by IB with anti-Myc and anti-Flag. (I) Schematic representation of plasmids for full-length (FL) and truncated RNF123. (J and K) HEK 293T cells were transfected with pCAGGS-SOCS1-Myc (1.5 mg/well) together with the plasmids for full-length or truncated RNF123 (1.5 mg/well). Thirty-six hours later, the cell lysates were immunoprecipitated with anti-Myc or anti-Flag, followed by IB with anti-Myc and anti-Flag. (L) HEK 293T cells were transfected with HA-Ub-K48 (1 mg/well) together with pCAGGS-SOCS1-Myc (1 mg/well) or pCAGGS-RNF123-Flag (1 mg/well) and pCAGGS-SOCS1-Myc. Thirty-six hours after transfection, the cell lysates were immunoprecipitated with anti-Myc, and ubiquitinated SOCS1 was detected by IB with anti-HA. (M) HEK 293T cells were transfected with pCAGGS-SOCS1-Myc (1 mg/well) and HA-Ub-K48 (1 mg/well) together with shRNA-RNF123 (1 mg/well) or shRNA-NC (blank control of shRNA-RNF123). Thirty-six hours after transfection, cells were treated with MG132 (10 μM) or DMSO for 6 h. The cell lysates were immunoprecipitated with anti-Myc, and ubiquitinated SOCS1 was detected by IB with anti-HA. (N) HEK 293T cells were transfected with pCAGGS-RNF123-Flag (1 mg/well) and HA-Ub-K48 (1 mg/well) together with the WT, the K114R or K137R single-point mutant, or the DM (1 mg/well). Thirty-six hours after transfection, the cell lysates were immunoprecipitated with anti-Myc, and ubiquitinated SOCS1 was detected by IB with anti-HA. All data are the mean values obtained from at least three replicates of independent experiments.
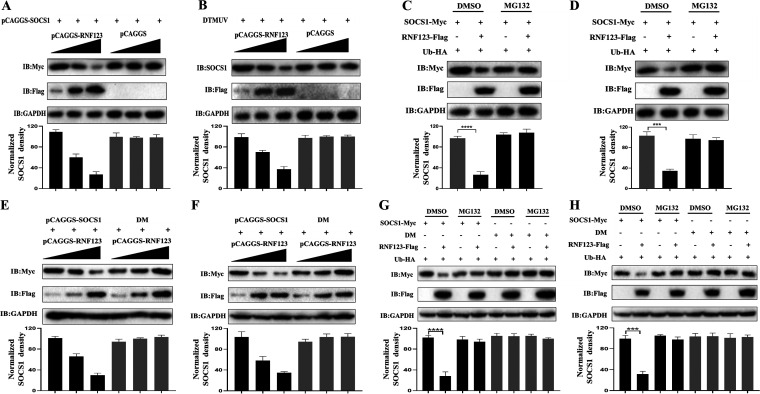
RNF123 mediates the proteasomal degradation of SOCS1.
Since K48-linked ubiquitination is often related to protein degradation events, we determined the level of SOCS1 protein when gradient doses of pCAGGS-RNF123-Flag and the same amounts of pCAGGS-SOCS1-Myc were cotransfected. The data showed that the endogenous and exogenous protein levels of SOCS1 were downregulated gradually with increasing RNF123 transfection doses (Fig. 3A and B). Moreover, we treated cells with MG132 or DMSO and found that when RNF123 was cotransfected with SOCS1, the protein levels of SOCS1 were significantly replenished in the MG132-treated group (Fig. 3C and D). Therefore, RNF123 mediates the proteasomal degradation of SOCS1. As shown in Fig. 3E and F, SOCS1 protein levels were dose-dependently downregulated when wild-type SOCS1 was cotransfected with gradient doses of RNF123 in both duck embryo fibroblasts (DEFs) and HEK 293T cells. However, when the DM was cotransfected with gradient doses of RNF123, the protein levels of SOCS1 with double-point mutations at K114 and K137 did not change significantly (Fig. 3E and F). As shown in Fig. 3G and H, when wild-type SOCS1 was cotransfected with RNF123, the protein level of SOCS1 in the MG132-treated group was replenished in both DEFs and HEK 293T cells. However, when the DM was cotransfected with RNF123, there was no significant difference in mutated SOCS1 protein levels between the DMSO-treated and MG132-treated groups in DEFs or HEK 293T cells (Fig. 3G and H). Therefore, RNF123 mainly mediates the ubiquitination of K114 and K137 of SOCS1, which causes the proteasomal degradation of SOCS1.
FIG 3.
RNF123 mediates the proteasomal degradation of SOCS1. (A) HEK 293T cells were transfected with gradient doses of pCAGGS-RNF123-Flag (0.5 mg, 1 mg, and 1.5 mg/well) together with pCAGGS (1.5 mg/well) or pCAGGS-SOCS1-Myc (1.5 mg/well). Thirty-six hours later, the protein level of exogenous SOCS1 was determined by IB with anti-Myc. (B) DEFs were transfected with gradient doses of pCAGGS-RNF123-Flag (0.5 mg, 1 mg, and 1.5 mg/well). Twenty-four hours later, cell samples were harvested by infecting DEFs with DTMUV at an MOI of 1 for 36 h. The protein level of endogenous SOCS1 was determined by IB with anti-SOCS1. (C and D) HEK 293T cells or DEFs were cotransfected with HA-Ub (1 mg/well), pCAGGS-SOCS1-Myc (1 mg/well), and pCAGGS-RNF123 (1 mg/well). Thirty-six hours later, cells were treated with MG132 (10 μM) or DMSO for 6 h. The protein level of SOCS1 was determined by IB with anti-Myc. (E and F) HEK 293T cells or DEFs were transfected with gradient doses of pCAGGS-RNF123-Flag (0.5 mg, 1 mg, and 1.5 mg/well) together with pCAGGS-SOCS1-Myc (1.5 mg/well) or the DM (1.5 mg/well). Thirty-six hours later, the protein level of SOCS1 was determined by IB with anti-Myc. (G and H) HEK 293T cells or DEFs were transfected with HA-Ub (1 mg/well) and pCAGGS-RNF123-Flag (1 mg/well) together with pCAGGS-SOCS1-Myc (1 mg/well) or the DM (1 mg/well). Thirty-six hours later, cells were treated with MG132 (10 μM) or DMSO for 6 h. The protein level of SOCS1 was determined by IB with anti-Myc. The densitometric values of the SOCS1 protein were quantified and analyzed. All data are the mean values obtained from at least three replicates of independent experiments. The t test was used to analyze significant differences between groups. ***, P < 0.001; ****, P < 0.0001.
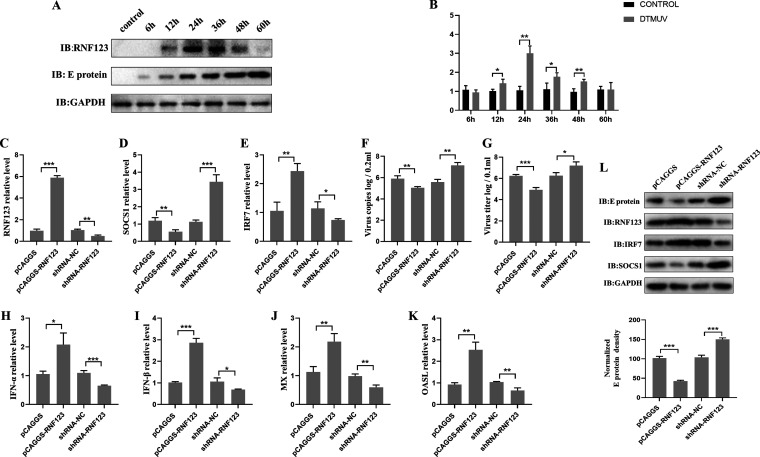
RNF123 promotes the production of type I IFN and inhibits viral replication.
It was shown previously that SOCS1 antagonizes DTMUV-induced type I IFN production by promoting the degradation of IRF7 (14), while in this study, we found that RNF123 mediates SOCS1 proteasome degradation, so we examined the effect of RNF123 on type I IFN signaling. First, the expression changes in RNF123 mRNA and protein in DEFs at different time points of DTMUV infection were examined. The data showed that compared with the control group, the levels of RNF123 protein and mRNA were significantly upregulated within 12 h to 36 h of DTMUV infection. However, at 48 h and 60 h of infection, the levels of RNF123 protein and mRNA were lower than those at 36 h of infection (Fig. 4A and B). Next, the effect of RNF123 on the expression of type I IFN and its downstream ISGs during DTMUV infection was examined. The data showed that the overexpression of RNF123 significantly upregulated the mRNA levels of type I IFNs and ISGs, whereas the opposite results were observed when RNF123 was knocked down (Fig. 4C and H to K). In addition, the effects of RNF123 on the SOCS1 and IRF7 mRNA and protein levels and viral replication were also examined. When RNF123 was overexpressed, the protein and mRNA levels of SOCS1, the virus copy number, the virus titer, and E protein expression were significantly downregulated (Fig. 4C, D, F, G, and L). However, the overexpression of RNF123 significantly upregulated the mRNA and protein levels of IRF7 (Fig. 4C, E, and L). When RNF123 was knocked down, the expression levels of the above-described genes showed an opposite trend. Taken together, these results show that RNF123 facilitates the expression of type I IFN and impairs DTMUV replication.
FIG 4.
RNF123 promotes the production of type I IFN and inhibits viral replication. (A and B) Cell samples were harvested by challenging DEFs with DTMUV at an MOI of 1 for 6 h, 12 h, 24 h, 36 h, 48 h, and 60 h. The protein level of RNF123 was determined by IB with anti-RNF123. The mRNA level of RNF123 was determined by RT-qPCR. (C to K) DEFs were transfected with pCAGGS (1.25 mg/well), pCAGGS-RNF123 (1.25 mg/well), shRNA-NC (1.25 mg/well), and shRNA-RNF123 (1.25 mg/well). Twenty-four hours later, the cell samples were harvested by challenging DEFs with DTMUV at an MOI of 1 for 36 h. RT-qPCR was used to determine the viral copy number and the mRNA levels of RNF123, SOCS1, IRF7, type I IFN, MX, and OASL, 2’, 5’-oligoadenylate synthetase-like protein. Cells were harvested for titer determination. (L) DEFs were transfected with pCAGGS (2.5 mg/well), pCAGGS-RNF123 (2.5 mg/well), shRNA-NC (2.5 mg/well), and shRNA-RNF123 (2.5 mg/well). Twenty-four hours later, the cell samples were harvested by challenging DEFs with DTMUV at an MOI of 1 for 36 h. The protein levels of RNF123, viral E protein, IRF7, and SOCS1 were determined by IB with anti-RNF123, anti-E, anti-IRF7, or anti-SOCS1, respectively. The densitometric values of the viral E protein in panel L were quantified and analyzed. All data are the mean values obtained from at least three replicates of independent experiments. The t test was used to analyze significant differences between groups. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
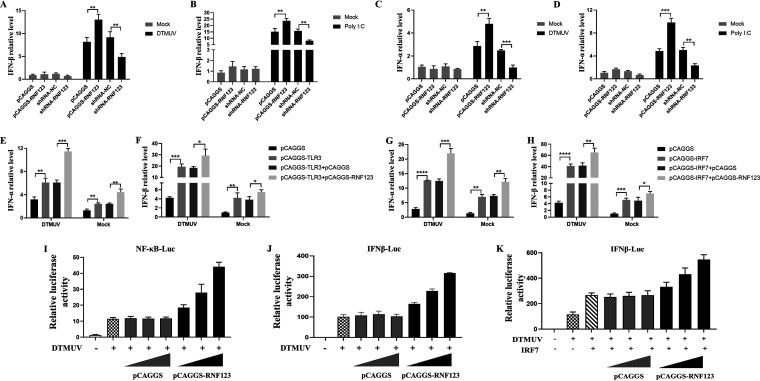
RNF123 promotes TLR3- and IRF7-mediated type I IFN production.
TLR3-mediated innate immunity is activated by DTMUV infection (12), and further research found that SOCS1 inhibits TLR3-mediated type I IFN expression by degrading IRF7, so the effect of RNF123 on TLR3- and IRF7-mediated type I IFN signaling were examined. As shown in Fig. 5, with DTMUV infection or poly(I:C) stimulation, RNF123 overexpression significantly upregulated type I IFN mRNA levels, while the knockdown of RNF123 significantly downregulated type I IFN mRNA levels (Fig. 5A to D). Furthermore, type I IFN mRNA levels were upregulated when TLR3 or IRF7 was overexpressed, whereas type I IFN mRNA levels were significantly upregulated when RNF123 was coexpressed with TLR3 or IRF7 compared with the controls (Fig. 5E to H). To further verify the above-described results, we transfected DEFs with gradient doses of RNF123 or an empty vector and challenged them with DTMUV. As shown in Fig. 5, DTMUV-induced NF-κB signaling and IFN-β signaling were dose-dependently upregulated with increasing doses of RNF123 (Fig. 5I and J). In addition, with increasing doses of RNF123 transfection, IRF7-mediated IFN-β signaling was also dose-dependently upregulated (Fig. 5K). Thus, during DTMUV infection, RNF123 promotes TLR3- and IRF7-mediated type I IFN signaling.
FIG 5.
RNF123 promotes TLR3- and IRF7-mediated type I IFN production. (A to D) DEFs were transfected with pCAGGS (1.25 mg/well), pCAGGS-RNF123 (1.25 mg/well), shRNA-NC (1.25 mg/well), and shRNA-RNF123 (1.25 mg/well). Twenty-four hours later, the cell samples were harvested by infecting DEFs with DTMUV at an MOI of 1 or stimulated with poly(I:C) for 36 h. RT-qPCR was used to determine the mRNA level of type I IFN. (E and F) DEFs were transfected with pCAGGS (0.625 mg/well), pCAGGS-TLR3 (0.625 mg/well), pCAGGS and pCAGGS-TLR3, and pCAGGS-RNF123 (0.625 mg/well) and pCAGGS-TLR3. Twenty-four hours later, the cell samples of uninfected DEFs or DEFs infected with DTMUV at an MOI of 1 for 36 h were harvested. RT-qPCR was used to determine the mRNA level of type I IFN. (G and H) DEFs were transfected with pCAGGS-IRF7 (0.625 mg/well), pCAGGS (0.625 mg/well), pCAGGS-RNF123 (0.625 mg/well) and pCAGGS-IRF7, and pCAGGS and pCAGGS-IRF7. Twenty-four hours later, the cell samples of uninfected DEFs or DEFs infected with DTMUV at an MOI of 1 for 36 h were harvested. RT-qPCR was used to determine the mRNA level of type I IFN. (I and J) DEFs were transfected with IFN-β–Luc or NF-κB–Luc (0.4 mg/well) and pRL-TK (0.04 mg/well) together with gradient doses of pCAGGS-RNF123 (0.1 mg, 0.2 mg, and 0.4 mg/well) or pCAGGS. Twenty-four hours after transfection, the cell samples were harvested for dual-luciferase assays by challenging DEFs with DTMUV at an MOI of 1 for 36 h (n = 5). (K) DEFs were cotransfected with pCAGGS-IRF7 (0.1 mg), IFN–β-Luc (0.4 mg/well), and pRL-TK (0.04 mg/well) together with gradient doses of pCAGGS or pCAGGS-RNF123 (0.1 mg, 0.2 mg, and 0.4 mg/well). Twenty-four hours after transfection, the cell samples were harvested for dual-luciferase assays by challenging DEFs with DTMUV for 36 h (n = 5). All data are the mean values obtained from at least three replicates of independent experiments. The t test was used to analyze significant differences between groups. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
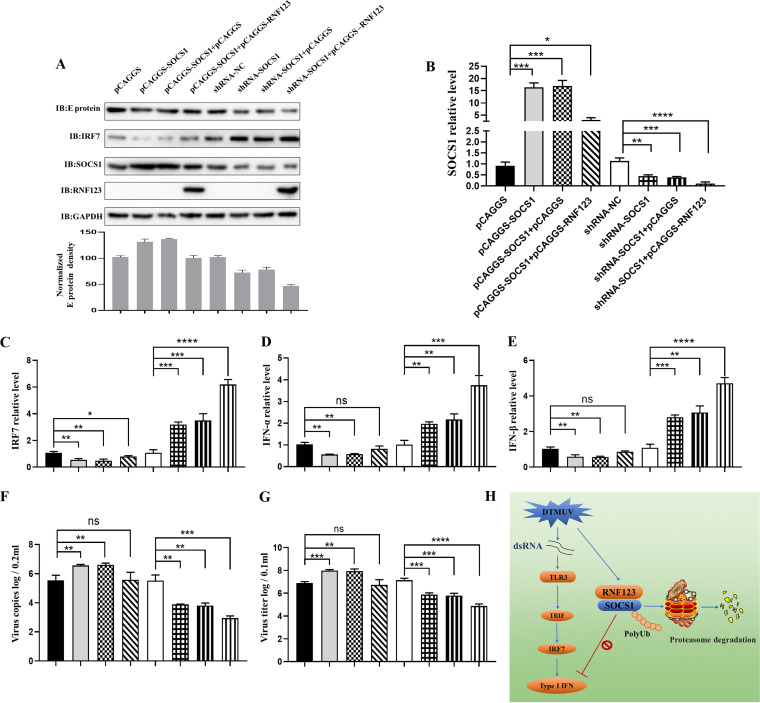
RNF123 affects type I IFN production and inhibits DTMUV proliferation via SOCS1.
In previous studies, we found that RNF123 combines with SOCS1 and mediates the ubiquitination and degradation of SOCS1 (Fig. 2 and 3). However, SOCS1 suppresses type I IFN expression and facilitates DTMUV replication by degrading IRF7 during DTMUV infection. Furthermore, we found that RNF123 promotes type I IFN expression during DTMUV infection (Fig. 4 and 5), so does RNF123 affect virus proliferation and type I IFN expression by degrading SOCS1? We therefore examined the effect of the overexpression of RNF123 on the viral copy number, the viral titer, and type I IFN when SOCS1 was knocked down or overexpressed. As shown in Fig. 6, when SOCS1 was knocked down, type I IFN mRNA levels and IRF7 mRNA and protein levels were upregulated (Fig. 6A to E), while the viral copy number, the viral titer, and E protein expression were downregulated (Fig. 6A, B, F, and G). Notably, cotransfection of RNF123 with shRNA-SOCS1 significantly upregulated type I IFN mRNA levels and IRF7 mRNA and protein levels compared with the control group (Fig. 6A to E), while the viral copy number, the viral titer, and E protein expression were significantly downregulated (Fig. 6A, B, F, and G). In addition, when SOCS1 was overexpressed, type I IFN mRNA levels and IRF7 mRNA and protein levels were downregulated (Fig. 6A to E), while the viral copy number, the viral titer, and E protein expression were upregulated (Fig. 6A, B, F, and G). However, when RNF123 was co-overexpressed with SOCS1, type I IFN mRNA levels, virus copy numbers, virus titers, and E protein expression were not significantly different from those in the control group (Fig. 6A, B, and D to G), while the mRNA and protein levels of IRF7 were complemented (Fig. 6A to C). Thus, these data suggest that RNF123 facilitates type I IFN production and antagonizes viral replication through SOCS1.
FIG 6.
RNF123 affects type I IFN production and inhibits DTMUV proliferation via SOCS1. DEFs were transfected with pCAGGS-SOCS1 (0.625 mg/well) and pCAGGS-RNF123-Flag (0.625 mg/well), pCAGGS-SOCS1, pCAGGS (0.625 mg/well), pCAGGS-SOCS1 and pCAGGS, shRNA-SOCS1 (0.625 mg/well) and pCAGGS-RNF123-Flag, shRNA-SOCS1 and pCAGGS, shRNA-SOCS1, and shRNA-NC (0.625 mg/well). Twenty-four hours later, the cell samples were harvested by infecting DEFs with DTMUV at an MOI of 1 for 36 h. (B to F) RT-qPCR was used to determine the viral copy numbers and the mRNA levels of SOCS1, IRF7, and type I IFN. (A) The protein levels of RNF123, viral E protein, IRF7, and SOCS1 were determined by IB with anti-RNF123, anti-E, anti-IRF7, or anti-SOCS1, respectively. (G) Cells were harvested for titer determination. (H) RNF123 mediates K48-linked ubiquitination and proteasomal degradation at K114 and K137 of SOCS1 to inhibit DTMUV replication. The TLR3-mediated innate immunity pathway is activated by DTMUV infection, thereby promoting IRF7-induced type I IFN expression. Previous studies demonstrated that SOCS1 inhibits type I IFN production during DTMUV infection by mediating the degradation of IRF7. In this study, it was shown that SOCS1 undergoes K48-linked ubiquitination at K114 and K137, and further studies found that RNF123 promotes the K48-linked ubiquitination and proteasomal degradation of SOCS1. Furthermore, RNF123 promoted TLR3- and IRF7-mediated type I IFN signaling by degrading SOCS1. However, we found that RNF123 was downregulated in the late stage of DTMUV infection, so DTMUV may suppress the production of type I IFN by antagonizing the expression of RNF123, thereby promoting its replication. dsRNA, double-stranded RNA. The densitometric values of the viral E protein in panel A were quantified and analyzed. All data are the mean values obtained from at least three replicates of independent experiments. The t test was used to analyze significant differences between groups. ns (not significant), P > 0.05; *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
DISCUSSION
Preinfection with DTMUV renders subsequent IFN therapy ineffective, suggesting that DTMUV has evolved some strategies to antagonize host IFN-mediated antiviral immune responses (21). Recent studies demonstrated that the expression of SOCS1 could be upregulated by DTMUV infection (22). Moreover, upregulated SOCS1 suppresses DTMUV-induced type I IFN signaling by degrading IRF7. However, SOCS1 undergoes K48-linked ubiquitination modification (14, 23). Further exploration found that JOSD1 prevents the degradation of SOCS1 by promoting the deubiquitination of SOCS1 (14). However, which E3 ubiquitin ligase catalyzes the K48-linked ubiquitination of SOCS1 remains unclear. In the present study, we demonstrate that RNF123 targets SOCS1 proteasomal degradation by facilitating the K48-linked ubiquitination of SOCS1 at K114 and K137, ultimately promoting TLR3/IRF7-mediated type I IFN expression and inhibiting DTMUV replication (Fig. 6H).
An excessive innate immune response can cause body damage; SOCS1 plays a vital role in the negative-feedback regulation of innate immunity, and many viruses use the negative regulation of the SOCS1 protein to suppress the host’s antiviral immune response. For example, hepatitis B virus inhibits TLR9-mediated IFN-α production by upregulating SOCS1 (24). Furthermore, Zika virus exploits the SOCS1 and SOCS3 proteins to inhibit RIG-I-like receptor (RLR)-mediated type I and III IFN production (25). However, studies on the PTM of SOCS1 seem to be rarely reported. Both JOSD1 and USP7 were identified as deubiquitinases of SOCS1 that stabilize SOCS1 expression by deubiquitinating SOCS1 (23, 26). However, which E3 ubiquitin ligase mediates SOCS1 ubiquitination and which lysine residues of SOCS1 are ubiquitinated have not been reported. In this study, we identified residues K114 and K137 of duck SOCS1 as target sites for K48-linked ubiquitination by co-IP experiments. Further mass spectrometry detection and co-IP revealed that the RING domain of the E3 ubiquitin ligase RNF123 is bound to the SH2 domain of SOCS1. In addition, the knockdown of RNF123 decreased the ubiquitination level of SOCS1, while the overexpression of RNF123 increased the ubiquitination level of SOCS1. Since K48-linked ubiquitination modification is mainly involved in protein degradation events, we examined whether RNF123 degrades SOCS1, and the results showed that RNF123 mediates the proteasomal degradation of SOCS1.
One of the components of the Kip1 ubiquitination-promoting complex (KPC) heterodimer is RNF123, so RNF123 is also known as KPC1, and another component of the KPC is UBAC1 (KPC2). Previous studies have shown that the KPC mediates the degradation of P27Kip1, a cyclin-dependent kinase inhibitor at the G0/G1-phase transition of the cell cycle. On the one hand, RNF123 recognizes and mediates the ubiquitination of P27. On the other hand, KPC2 binds to P27 and mediates the proteasomal degradation of P27 (27–29). Recent studies have also found that RNF123 inhibits tumor growth by promoting the ubiquitination and proteasomal degradation of NF-κB1 (30). We found that in the early stage of DTMUV infection, RNF123 was upregulated, and the overexpression of RNF123 promoted the expression of type I IFN and downstream antiviral ISGs but inhibited DTMUV replication. Since the TLR3/IRF7-mediated innate immunity pathway is activated by DTMUV infection, we determined the effect of RNF123 on this pathway signal. The results showed that RNF123 promoted TLR3/IRF7-induced type I IFN signaling and inhibited viral replication by degrading SOCS1. Although previous studies have shown that human RNF123 negatively regulates RLR signaling-mediated antiviral signaling in an E3 ubiquitin ligase-independent manner (31), many studies have shown that the RNF protein family is more likely to function as E3 ubiquitin ligases to regulate innate immunity pathways and may be involved in regulation through two distinct mechanisms. For example, RNF26 plays a dual role in the regulation of virus-induced type I IFN. On the one hand, RNF26 inhibits the degradation of MITA by RNF5 and promotes type I IFN signaling by mediating the ubiquitination of the K150 site of MITA, which is also the ubiquitination target site of RNF5; on the other hand, RNF26 facilitates the autophagic degradation of IRF3 to prevent damage caused by the excessive production of type I IFN (32). Furthermore, it was found that RNF115 constitutively catalyzes the K48-linked ubiquitination and proteasomal degradation of MAVS in uninfected cells, while in herpes simplex virus 1 (HSV-1)-infected cells, RNF115 binds to STING and boosts the K63-linked ubiquitination of STING (33). Although our study elucidates that RNF123 promotes type I IFN signaling during DTMUV infection by promoting the ubiquitinated degradation of SOCS1, the specific role of this pathway in viral pathogenesis needs to be further clarified in vivo. Moreover, the mechanism of the downregulation of RNF123 in the late stage of DTMUV infection remains to be further explored.
Collectively, our studies reveal that RNF123 binds to the SH2 domain of SOCS1 through its RING domain and promotes the K48-linked ubiquitination of the K114 and K137 residues of SOCS1, resulting in the proteasomal degradation of SOCS1. Further studies demonstrated that during DTMUV infection, RNF123 promoted TLR3/IRF7-induced type I IFN expression by degrading SOCS1, thereby inhibiting viral replication (Fig. 6H).
MATERIALS AND METHODS
Ethics statement.
The use of duck embryos in this study was approved by the Animal Ethics Committee of Sichuan Agricultural University (approval no. 2015-016), and we followed the animal experiment guidelines of the National Institutes of Health.
Cells and virus.
Duck embryo fibroblasts (DEFs) and human embryonic kidney HEK 293T cells were grown for 12 to 24 h in Dulbecco’s modified Eagle’s medium (DMEM) (catalog no. 12800-058; Gibco, USA) supplemented with 10% fetal bovine serum (FBS) (Gibco, USA) at 37°C with 5% CO2. The DTMUV CQW1 strain (GenBank accession no. KM233707.1) was used in this study, and the viral titer of this strain was 6.1 × 10−6 tissue culture infective doses (TCID50)/100 μL.
Plasmids and reagents.
HA-ubiquitin (HA-Ub), HA-Ub-K48, plasmids for full-length and mutant SOCS1, pRL-TK, IFN-β–Luc, NF-κB–Luc, anti-duck SOCS1 polyclonal antibody, anti-duck IRF7 polyclonal antibody, and anti-E monoclonal antibody were provided by our laboratory. The point mutation primers (as shown in Table 1) were designed according to the manufacturer’s instructions (FM111; Transgen Biotech, China) to construct the 13 lysine single-point-mutation plasmids for SOCS1 and the K114,K137 double-point-mutation plasmid. We designed specific primers (as shown in Table 2) for duck RNF123 and RNF123 domain deletion mutant overexpression vectors based on the sequences in GenBank and cloned them into the pCAGGS expression vector by homologous recombination. GenePharma (Shanghai, China) synthesized shRNA-RNF123 and shRNA-SOCS1 used in this study. Abs against Myc (clone 71D10; Cell Signaling Technology [CST], USA), HA (MBL, Japan), Flag (D6W5B; CST, USA), RNF123 (catalog no. DF9488; Affinity Biosciences, China), glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Proteintech, China), and β-actin (Proteintech, China) were used in this study.
TABLE 1.
Primers used to construct SOCS1 point mutant plasmids
| Primer name | Sequence (5′–3′) |
|---|---|
| K6R F | ATGGTAGCGCACAGCAGGGTGTCAGCAGA |
| K6R R | CTGCTGTGCGCTACCATCAGATCCTCTTC |
| K88R F | AGTGCTGCCCACGAGAGGCTGAAGTCTGA |
| K88R R | CTCTCGTGGGCAGCACTAACAGTCAGAGG |
| K90R F | GCCCACGAGAAGCTGAGGTCTGAGCCCGA |
| K90R R | CTCAGCTTCTCGTGGGCAGCACTAACAGTC |
| K105R F | CAGGGACAGCACGCAGAGGAATTGTTTCTT |
| K105R R | CTCTGCGTGCTGTCCCTGATGAGGAAGGT |
| K114R F | TTTGCCATCAGTGTTAGGACTGCAACTG |
| K114R R | CTAACACTGATGGCAAAGAAACAATTCTTC |
| K137R F | AGCCTGGATGGCAGCAGAGAGACTTTTGAC |
| K137R R | CTGCTGCCATCCAGGCTGAAACGCCCA |
| K145R F | TTTGACTGTCTCTTCAGGCTGCTGGAACA |
| K145R R | CTGAAGAGACAGTCAAAAGTCTCTTTGCT |
| K156R F | TTAAGCTCCCCGAGGAGGGTACTGGTTAC |
| K156R R | CTCCTCGGGGAGCTTAAGTAATGTTCCAG |
| K164R F | GTTACCCCCCTGCGCAGAGTCCGTGTGCA |
| K164R R | CTGCGCAGGGGGGTAACCAGTACCTTCCT |
| K176R F | CAGGAGCTCTGTCGGAGGAGCATTGTGAAG |
| K176R R | CTCCGACAGAGCTCCTGCAAGGGCTGCA |
| K180R F | CGGAAGAGCATTGTGAGGACTTTTGGAAG |
| K180R R | CTCACAATGCTCTTCCGACAGAGCTCCT |
| K197R F | CTCAATCCTGTTTTAAGGGACTACCTGAA |
| K197R R | CTTAAAACAGGATTGAGAGGGATTTGGTT |
| K201R F | TTAAAGGACTACCTGAGATCATTCCCATT |
| K201R R | CTCAGGTAGTCCTTTAAAACAGGATTGA |
TABLE 2.
Primers for the construction of plasmids for full-length and truncated RNF123
| Primer name | Sequence (5′–3′) |
|---|---|
| pCAGGS-RNF123-Flag F | CTGTCTCATCATTTTGGCAAAGAATTCGCCACCATGGGCATGACGTCGCGAGGTGCTGGG |
| pCAGGS-RNF123-Flag R | TGCTAGCTCGAGGCATGCCCGGGTACCTTACTTATCGTCGTCATCCTTGTAATCCGAGCTGGCGGGCTTGGTGAAGT |
| RNF123(293–1250) F | CTGTCTCATCATTTTGGCAAAGAATTCGCCACCATGGTGACGGAGGGGAAGCTGGTGGAG |
| RNF123(293–1250) R | TGCTAGCTCGAGGCATGCCCGGGTACCTTACTTATCGTCGTCATCCTTGTAATCGCTGGTGGGCAGCATGGT |
| RNF123(1072–1308) F | CTGTCTCATCATTTTGGCAAAGAATTCGCCACCATGGGCTGTGCCACCTGCTTCGACCTGTCGGTG |
| RNF123(1–1057) R | TGCTAGCTCGAGGCATGCCCGGGTACCTTACTTATCGTCGTCATCCTTGTAATCCTCCGCTGCCTGCTGGATCTCCTG |
| RNF123(1–1071) R | TGCTAGCTCGAGGCATGCCCGGGTACCTTACTTATCGTCGTCATCCTTGTAATCCACCTTCAGCTGGCGGCTGTCCAC |
Mass spectrometry analysis.
SDS-PAGE gels were stained with Coomassie brilliant blue, cut into 1- by 1-cm gel blocks, and sent to PTM Bio (China) for mass spectrometry analysis. The data were processed using Proteome Discoverer 1.3. Tandem mass spectra were searched against the Anas platyrhynchos database.
RNA isolation and quantitative real-time PCR (qRT-PCR) analysis.
The total RNA from cells in each well was extracted using RNAiso plus reagent. RNA was then reverse transcribed to cDNA using PrimeScript RT master mix (TaKaRa, Japan), according to the manufacturer’s instructions. Using the quantitative primers in Table 3, quantitative PCR (qPCR) was performed by using the Bio-Rad CFX96 real-time detection system to determine the mRNA levels of target genes. The relative mRNA level of each target gene was calculated using the comparative threshold cycle (CT) (2−ΔΔCT) method with β-actin as the internal reference.
TABLE 3.
RT-qPCR primers used in the present study
| Primer name | Sequence (5′–3′) |
|---|---|
| RNF123-F | TCAGGAGGAAGGAGTTG |
| RNF123-R | TTGCCATAGTTGGTAGTG |
| SOCS1 F | CTTGCTGGATGCCTGTGG |
| SOCS1 R | CTGCGTGCTGTCCCTGAT |
| DTMUV-E F | AATGGCTGTGGCTTGTTTGG |
| DTMUV-E R | GGGCGTTATCACGAATCTA |
| β-actin F | CCGGGCATCGCTGACA |
| β-actin R | GGATTCATCATACTCCTGCTTTGCT |
| IFN-α F | CCACCATGCCTGGGCCATCAG |
| IFN-α R | AGGAGAAGGCGTTGGCGGGAG |
| IFN-β F | CGCCTGGACACGCTAATA |
| IFN-β R | AGCTGGTGCCTCTTGCTC |
| IRF7 F | CGCCACCCGCCTGAAGAAGT |
| IRF7 R | CTGCCCGAAGCAGAGGAAGAT |
| MX F | CCTAAGGGAGAAAGGACACT |
| MX R | GACCACGACACTTCACAACC |
| OASL F | GCAGGCAGAGGCTGTCGTTC |
| OASL R | ATGGACTCGCCGTTGGAGGA |
Indirect immunofluorescence.
DEFs were inoculated into slides in 12-well plates. When the cell confluence reached about 90%, the cells were infected with DTMUV at a multiplicity of infection (MOI) of 1. Thirty-six hours after infection, the cells were collected for indirect immunofluorescence detection according to previously described methods (14).
Dual-luciferase activity assays.
When the DEF cells reached 70% confluence, the NF-κB–Luc (400 ng/well) or IFN-β–Luc (400 ng/well) luciferase reporter plasmid and the internal reference plasmid pRL-TK (40 ng/well) were mixed with gradient amounts of pCAGGS-RNF123 or the control plasmid pCAGGS and cotransfected into cells. Twenty-four hours after transfection, DEFs were challenged with DTMUV at an MOI of 1 for 36 h, and the luciferase activity of the target gene was detected using the Dual-Glo luciferase assay system (Promega) according to the manufacturer’s instructions. The ratios of firefly luciferase activity to Renilla luciferase activity are the normalized data obtained.
Co-IP, Western blot analysis, and ubiquitination assay.
Thirty-six hours after transfection, DEFs or HEK 293T cells were lysed on ice for 30 min using IP lysis buffer (Thermo Fisher Scientific) and then centrifuged at 13,000 × g for 10 min at 4°C to remove cell debris and obtain the supernatant. For IP experiments, 50 μL of the lysate was taken as the input group, and the remaining 550 μL of the lysate was added with the specified Abs according to the manufacturer’s instructions and incubated at 4°C for 12 to 24 h. SureBeads protein G (Bio-Rad) was then added to each sample, and the samples were incubated for 4 to 6 h. Next, after washing the beads three times, SDS-PAGE was performed, followed by immunoblot (IB) analysis using specific Abs. The detection of ubiquitination was performed as previously described (14, 34).
Virus titer determination.
Twenty-four hours after transfection, DEFs were challenged with DTMUV at an MOI of 1 for 36 h, and the virus titer was determined as described previously (22, 35).
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (32172833), the Natural Science Foundation of Sichuan Province (2022NSFSC0078), the earmarked fund for the China Agriculture Research System of MOF and MARA, and the Sichuan Veterinary Medicine and Drug Innovation Group of the China Agricultural Research System (SCCXTD-2021-18).
Contributor Information
Anchun Cheng, Email: chenganchun@vip.163.com.
Renyong Jia, Email: jiary@sicau.edu.cn.
Bryan R. G. Williams, Hudson Institute of Medical Research
REFERENCES
- 1.Su J, Li S, Hu X, Yu X, Wang Y, Liu P, Lu X, Zhang G, Hu X, Liu D, Li X, Su W, Lu H, Mok NS, Wang P, Wang M, Tian K, Gao GF. 2011. Duck egg-drop syndrome caused by BYD virus, a new Tembusu-related flavivirus. PLoS One 6:e18106. doi: 10.1371/journal.pone.0018106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yan P, Zhao Y, Zhang X, Xu D, Dai X, Teng Q, Yan L, Zhou J, Ji X, Zhang S, Liu G, Zhou Y, Kawaoka Y, Tong G, Li Z. 2011. An infectious disease of ducks caused by a newly emerged Tembusu virus strain in mainland China. Virology 417:1–8. doi: 10.1016/j.virol.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 3.Liu P, Lu H, Li S, Moureau G, Deng Y-Q, Wang Y, Zhang L, Jiang T, de Lamballerie X, Qin C-F, Gould EA, Su J, Gao GF. 2012. Genomic and antigenic characterization of the newly emerging Chinese duck egg-drop syndrome flavivirus: genomic comparison with Tembusu and Sitiawan viruses. J Gen Virol 93:2158–2170. doi: 10.1099/vir.0.043554-0. [DOI] [PubMed] [Google Scholar]
- 4.Liu P, Lu H, Li S, Wu Y, Gao GF, Su J. 2013. Duck egg drop syndrome virus: an emerging Tembusu-related flavivirus in China. Sci China Life Sci 56:701–710. doi: 10.1007/s11427-013-4515-z. [DOI] [PubMed] [Google Scholar]
- 5.Tang Y, Diao Y, Chen H, Ou Q, Liu X, Gao X, Yu C, Wang L. 2015. Isolation and genetic characterization of a Tembusu virus strain isolated from mosquitoes in Shandong, China. Transbound Emerg Dis 62:209–216. doi: 10.1111/tbed.12111. [DOI] [PubMed] [Google Scholar]
- 6.Wang H-J, Li X-F, Liu L, Xu Y-P, Ye Q, Deng Y-Q, Huang X-Y, Zhao H, Qin E-D, Shi P-Y, Gao GF, Qin C-F. 2016. The emerging duck flavivirus is not pathogenic for primates and is highly sensitive to mammalian interferon antiviral signaling. J Virol 90:6538–6548. doi: 10.1128/JVI.00197-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Guinn ML, Turell MJ, Kengluecha A, Jaichapor B, Kankaew P, Miller RS, Endy TP, Jones JW, Coleman RE, Lee JS. 2013. Field detection of Tembusu virus in western Thailand by RT-PCR and vector competence determination of select Culex mosquitoes for transmission of the virus. Am J Trop Med Hyg 89:1023–1028. doi: 10.4269/ajtmh.13-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ti J, Zhang M, Li Z, Li X, Diao Y. 2016. Duck Tembusu virus exhibits pathogenicity to Kunming mice by intracerebral inoculation. Front Microbiol 7:190. doi: 10.3389/fmicb.2016.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang Y, Gao X, Diao Y, Feng Q, Chen H, Liu X, Ge P, Yu C. 2013. Tembusu virus in human, China. Transbound Emerg Dis 60:193–196. doi: 10.1111/tbed.12085. [DOI] [PubMed] [Google Scholar]
- 10.Saito T, Gale M, Jr. 2007. Principles of intracellular viral recognition. Curr Opin Immunol 19:17–23. doi: 10.1016/j.coi.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 11.Thompson AJ, Locarnini SA. 2007. Toll-like receptors, RIG-I-like RNA helicases and the antiviral innate immune response. Immunol Cell Biol 85:435–445. doi: 10.1038/sj.icb.7100100. [DOI] [PubMed] [Google Scholar]
- 12.Guo H, Cheng A, Zhang X, Pan Y, Wang M, Huang J, Zhu D, Chen S, Liu M, Zhao X, Wu Y, Yang Q, Zhang S, Yu Y, Pan L, Tian B, Rehman MU, Chen X, Liu Y, Zhang L, Yin Z, Jing B, Jia R. 2020. DEF cell-derived exosomal miR-148a-5p promotes DTMUV replication by negative regulating TLR3 expression. Viruses 12:94. doi: 10.3390/v12010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen S, Wang T, Liu P, Yang C, Wang M, Jia R, Zhu D, Liu M, Yang Q, Wu Y, Zhao X, Cheng A. 2019. Duck interferon regulatory factor 7 (IRF7) can control duck Tembusu virus (DTMUV) infection by triggering type I interferon production and its signal transduction pathway. Cytokine 113:31–38. doi: 10.1016/j.cyto.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 14.Huang S, Huang J, Cui M, Wu X, Wang M, Zhu D, Chen S, Liu M, Zhao X, Wu Y, Yang Q, Zhang S, Ou X, Mao S, Gao Q, Yu Y, Tian B, Liu Y, Zhang L, Yin Z, Jing B, Chen X, Cheng A, Jia R. 2022. Duck Tembusu virus inhibits type I interferon production through the JOSD1-SOCS1-IRF7 negative-feedback regulation pathway. J Virol 96:e00930-22. doi: 10.1128/jvi.00930-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo X, Chen X-X, Qiao S, Li R, Xie S, Zhou X, Deng R, Zhou E-M, Zhang G. 2020. Porcine reproductive and respiratory syndrome virus enhances self-replication via AP-1-dependent induction of SOCS1. J Immunol 204:394–407. doi: 10.4049/jimmunol.1900731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olière S, Hernandez E, Lézin A, Arguello M, Douville R, Nguyen TL, Olindo S, Panelatti G, Kazanji M, Wilkinson P, Sékaly RP, Césaire R, Hiscott J. 2010. HTLV-1 evades type I interferon antiviral signaling by inducing the suppressor of cytokine signaling 1 (SOCS1). PLoS Pathog 6:e1001177. doi: 10.1371/journal.ppat.1001177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uetani K, Hiroi M, Meguro T, Ogawa H, Kamisako T, Ohmori Y, Erzurum SC. 2008. Influenza A virus abrogates IFN-gamma response in respiratory epithelial cells by disruption of the Jak/Stat pathway. Eur J Immunol 38:1559–1573. doi: 10.1002/eji.200737045. [DOI] [PubMed] [Google Scholar]
- 18.Kong Z, Yin H, Wang F, Liu Z, Luan X, Sun L, Liu W, Shang Y. 2022. Pseudorabies virus tegument protein UL13 recruits RNF5 to inhibit STING-mediated antiviral immunity. PLoS Pathog 18:e1010544. doi: 10.1371/journal.ppat.1010544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun Y, Zheng H, Yu S, Ding Y, Wu W, Mao X, Liao Y, Meng C, Ur Rehman Z, Tan L, Song C, Qiu X, Wu F, Ding C. 2019. Newcastle disease virus V protein degrades mitochondrial antiviral signaling protein to inhibit host type I interferon production via E3 ubiquitin ligase RNF5. J Virol 93:e00322-19. doi: 10.1128/JVI.00322-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiang Y, Zhang W, Jia P, Lu X, Liu W, Yi M, Jia K. 2021. E3 ubiquitin ligase RNF114 inhibits innate immune response to red-spotted grouper nervous necrosis virus infection in sea perch by targeting MAVS and TRAF3 to mediate their degradation. J Immunol 206:77–88. doi: 10.4049/jimmunol.2000083. [DOI] [PubMed] [Google Scholar]
- 21.Zhang W, Jiang B, Zeng M, Duan Y, Wu Z, Wu Y, Wang T, Wang M, Jia R, Zhu D, Liu M, Zhao X, Yang Q, Wu Y, Zhang S, Liu Y, Zhang L, Yu Y, Pan L, Chen S, Cheng A. 2020. Binding of duck Tembusu virus nonstructural protein 2A to duck STING disrupts induction of its signal transduction cascade to inhibit beta interferon induction. J Virol 94:e01850-19. doi: 10.1128/JVI.01850-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang S, Cheng A, Cui M, Pan Y, Wang M, Huang J, Zhu D, Chen S, Liu M, Zhao X, Wu Y, Yang Q, Zhang S, Ou X, Mao S, Yu Y, Tian B, Liu Y, Zhang L, Yin Z, Jing B, Chen X, Jia R. 2020. Duck Tembusu virus promotes the expression of suppressor of cytokine signaling 1 by downregulating miR-148a-5p to facilitate virus replication. Infect Genet Evol 85:104392. doi: 10.1016/j.meegid.2020.104392. [DOI] [PubMed] [Google Scholar]
- 23.Wang X, Zhang L, Zhang Y, Zhao P, Qian L, Yuan Y, Liu J, Cheng Q, Xu W, Zuo Y, Guo T, Yu Z, Zheng H. 2017. JOSD1 negatively regulates type-I interferon antiviral activity by deubiquitinating and stabilizing SOCS1. Viral Immunol 30:342–349. doi: 10.1089/vim.2017.0015. [DOI] [PubMed] [Google Scholar]
- 24.Xu Y, Hu Y, Shi B, Zhang X, Wang J, Zhang Z, Shen F, Zhang Q, Sun S, Yuan Z. 2009. HBsAg inhibits TLR9-mediated activation and IFN-alpha production in plasmacytoid dendritic cells. Mol Immunol 46:2640–2646. doi: 10.1016/j.molimm.2009.04.031. [DOI] [PubMed] [Google Scholar]
- 25.Seong R-K, Lee JK, Shin OS. 2020. Zika virus-induction of the suppressor of cytokine signaling 1/3 contributes to the modulation of viral replication. Pathogens 9:163. doi: 10.3390/pathogens9030163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yuan Y, Miao Y, Zeng C, Liu J, Chen X, Qian L, Wang X, Qian F, Yu Z, Wang J, Qian G, Fu Q, Lv H, Zheng H. 2020. Small-molecule inhibitors of ubiquitin-specific protease 7 enhance type-I interferon antiviral efficacy by destabilizing SOCS1. Immunology 159:309–321. doi: 10.1111/imm.13147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kamura T, Hara T, Matsumoto M, Ishida N, Okumura F, Hatakeyama S, Yoshida M, Nakayama K, Nakayama KI. 2004. Cytoplasmic ubiquitin ligase KPC regulates proteolysis of p27(Kip1) at G1 phase. Nat Cell Biol 6:1229–1235. doi: 10.1038/ncb1194. [DOI] [PubMed] [Google Scholar]
- 28.Kotoshiba S, Kamura T, Hara T, Ishida N, Nakayama KI. 2005. Molecular dissection of the interaction between p27 and Kip1 ubiquitylation-promoting complex, the ubiquitin ligase that regulates proteolysis of p27 in G1 phase. J Biol Chem 280:17694–17700. doi: 10.1074/jbc.M500866200. [DOI] [PubMed] [Google Scholar]
- 29.Hara T, Kamura T, Kotoshiba S, Takahashi H, Fujiwara K, Onoyama I, Shirakawa M, Mizushima N, Nakayama KI. 2005. Role of the UBL-UBA protein KPC2 in degradation of p27 at G1 phase of the cell cycle. Mol Cell Biol 25:9292–9303. doi: 10.1128/MCB.25.21.9292-9303.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kravtsova-Ivantsiv Y, Shomer I, Cohen-Kaplan V, Snijder B, Superti-Furga G, Gonen H, Sommer T, Ziv T, Admon A, Naroditsky I, Jbara M, Brik A, Pikarsky E, Kwon YT, Doweck I, Ciechanover A. 2015. KPC1-mediated ubiquitination and proteasomal processing of NF-κB1 p105 to p50 restricts tumor growth. Cell 161:333–347. doi: 10.1016/j.cell.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 31.Wang S, Yang Y-K, Chen T, Zhang H, Yang W-W, Song S-S, Zhai Z-H, Chen D-Y. 2016. RNF123 has an E3 ligase-independent function in RIG-I-like receptor-mediated antiviral signaling. EMBO Rep 17:1155–1168. doi: 10.15252/embr.201541703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qin Y, Zhou M-T, Hu M-M, Hu Y-H, Zhang J, Guo L, Zhong B, Shu H-B. 2014. RNF26 temporally regulates virus-triggered type I interferon induction by two distinct mechanisms. PLoS Pathog 10:e1004358. doi: 10.1371/journal.ppat.1004358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Z-D, Xiong T-C, Yao S-Q, Wei M-C, Chen M, Lin D, Zhong B. 2020. RNF115 plays dual roles in innate antiviral responses by catalyzing distinct ubiquitination of MAVS and MITA. Nat Commun 11:5536. doi: 10.1038/s41467-020-19318-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu C-F, Peng W-M, Schlee M, Barchet W, Eis-Hübinger A-M, Kolanus W, Geyer M, Schmitt S, Steinhagen F, Oldenburg J, Novak N. 2018. SOCS1 and SOCS3 target IRF7 degradation to suppress TLR7-mediated type I IFN production of human plasmacytoid dendritic cells. J Immunol 200:4024–4035. doi: 10.4049/jimmunol.1700510. [DOI] [PubMed] [Google Scholar]
- 35.Reed LJ, Muench H. 1938. A simple method of estimating fifty per cent endpoints. Am J Hyg 27:493–497. [Google Scholar]