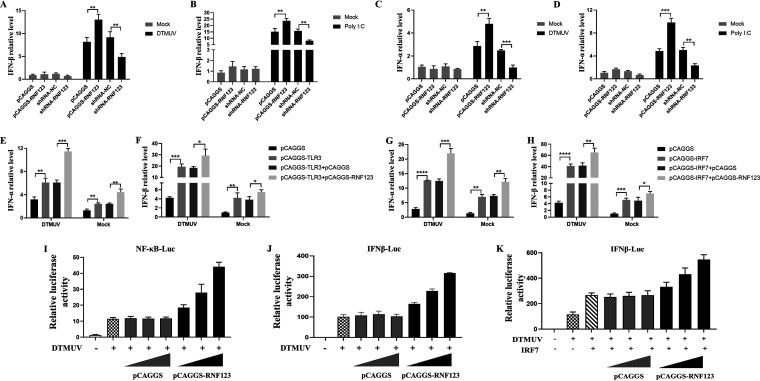
FIG 5.
RNF123 promotes TLR3- and IRF7-mediated type I IFN production. (A to D) DEFs were transfected with pCAGGS (1.25 mg/well), pCAGGS-RNF123 (1.25 mg/well), shRNA-NC (1.25 mg/well), and shRNA-RNF123 (1.25 mg/well). Twenty-four hours later, the cell samples were harvested by infecting DEFs with DTMUV at an MOI of 1 or stimulated with poly(I:C) for 36 h. RT-qPCR was used to determine the mRNA level of type I IFN. (E and F) DEFs were transfected with pCAGGS (0.625 mg/well), pCAGGS-TLR3 (0.625 mg/well), pCAGGS and pCAGGS-TLR3, and pCAGGS-RNF123 (0.625 mg/well) and pCAGGS-TLR3. Twenty-four hours later, the cell samples of uninfected DEFs or DEFs infected with DTMUV at an MOI of 1 for 36 h were harvested. RT-qPCR was used to determine the mRNA level of type I IFN. (G and H) DEFs were transfected with pCAGGS-IRF7 (0.625 mg/well), pCAGGS (0.625 mg/well), pCAGGS-RNF123 (0.625 mg/well) and pCAGGS-IRF7, and pCAGGS and pCAGGS-IRF7. Twenty-four hours later, the cell samples of uninfected DEFs or DEFs infected with DTMUV at an MOI of 1 for 36 h were harvested. RT-qPCR was used to determine the mRNA level of type I IFN. (I and J) DEFs were transfected with IFN-β–Luc or NF-κB–Luc (0.4 mg/well) and pRL-TK (0.04 mg/well) together with gradient doses of pCAGGS-RNF123 (0.1 mg, 0.2 mg, and 0.4 mg/well) or pCAGGS. Twenty-four hours after transfection, the cell samples were harvested for dual-luciferase assays by challenging DEFs with DTMUV at an MOI of 1 for 36 h (n = 5). (K) DEFs were cotransfected with pCAGGS-IRF7 (0.1 mg), IFN–β-Luc (0.4 mg/well), and pRL-TK (0.04 mg/well) together with gradient doses of pCAGGS or pCAGGS-RNF123 (0.1 mg, 0.2 mg, and 0.4 mg/well). Twenty-four hours after transfection, the cell samples were harvested for dual-luciferase assays by challenging DEFs with DTMUV for 36 h (n = 5). All data are the mean values obtained from at least three replicates of independent experiments. The t test was used to analyze significant differences between groups. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.