Abstract
Background
Neisseria gonorrhoeae (NG) has developed antimicrobial resistance (AMR) to multiple classes of antibiotics. While treatment of symptomatic NG in groups, such as men who have sex with men (MSM), is crucial, screening programs targeting asymptomatic NG cases may contribute to excessive antibiotic exposure of the population and thus to the emergence of gonococcal AMR. Our primary aim was to assess if intense screening could promote AMR in NG.
Methods
We built a network-based model of NG transmission dynamics among MSM in Belgium to estimate the prevalence of NG in the population and the risk of AMR. The model simulates daily transmission of NG among 3 anatomical sites in a population of 10 000 MSM, grouped as low risk or high risk, over 10 years. The effect of group-wise variation in treatment efficacy levels and screening intensities on NG prevalence and cumulative risk of AMR emergence was evaluated.
Results
Increasing screening intensity in the low-risk MSM had little effect on NG prevalence. An inverse correlation between screening intensity in the high-risk group and both NG prevalence and the risk for azithromycin resistance was observed, irrespective of the screening intensity in the low-risk group. High-risk MSM were at higher risk for azithromycin-resistant NG in all screening intensity and treatment efficacy scenarios, compared to low-risk MSM.
Conclusions
Our results suggest that intensive screening in the low-risk population has little impact on prevalence but may increase the probability of AMR emerging. In contrast, intensive screening in the high-risk population reduces both the prevalence of NG and macrolide resistance.
Keywords: antimicrobial resistance, mathematical model, men who have sex with men, Neisseria gonorrhoeae
Screening for gonorrhea decreases the prevalence of Neisseria gonorrhoeae; however, screening among low-risk men who have sex with men has little effect on gonorrhea prevalence. Intensive screening in the high-risk population reduces both the prevalence of N gonorrhoeae and macrolide resistance.
The incidence of Neisseria gonorrhoeae (NG) is increasing in many European countries and elsewhere [1, 2]. Around half of the reported gonorrhea cases in European countries (48%) [1] and the United States (47%) [2] are attributed to men who have sex with men (MSM). One of the responses to this increase has been to intensify screening for NG among persons with high-risk behavior [3]. Several national and international guidelines recommend at least annual screening for gonorrhea and chlamydia (Chlamydia trachomatis) for sexually active MSM, and every 3–6 months in those at highest risk [3–9]. A concern with intensified screening is that it may accelerate the acquisition and spread of antimicrobial resistance (AMR). NG has developed resistance to all classes of antibiotics it has been exposed to, including the currently recommended therapies [10–12]. Currently, dual treatment with a combination of ceftriaxone and azithromycin is the recommended treatment for gonorrhea in many countries including Belgium [13], whereas other countries advocate ceftriaxone monotherapy [14]. Intensive screening in MSM has been found to result in macrolide consumption levels in excess of thresholds associated with the induction of resistance in a number of important pathogens [15–17].
No randomized clinical trials have evaluated if screening MSM for NG has an effect on NG prevalence or the emergence of AMR. A systematic review of observational studies found that screening did not have a discernable effect on the prevalence of NG [18]. A number of individual- and ecological-level analyses of NG surveillance or self-reported NG diagnoses data have likewise found that NG screening was not associated with a decrease in NG incidence [19, 20]. Several modeling studies have investigated the effect of screening intensity on NG prevalence and incidence [21–25]. While most of these studies found a strong inverse correlation between screening intensity and NG prevalence [22, 23, 25], none of them have included the impact of screening on the probability of AMR emerging in NG [21, 24, 25]. Likewise, a number of modeling studies have included the aspect of antibiotic resistance in NG among MSM but have not included the impact of sexually transmitted infection (STI) screening [26, 27].
Furthermore, few of these modeling studies have used network or individual-based models of gonorrhea transmission [21, 22, 28]. Network models allow for a more complex and realistic structure of the contact network and behavioral characteristics (eg, risk group, condom use) and are able to account for all 3 anatomical sites of NG infection among MSM (oropharynx, urethra, and rectum). Here, we describe a network model of gonorrhea transmission in an MSM population in Belgium. Our primary aim was to assess if different intensities of screening could induce antibiotic resistance in N gonorrhoeae and, if so, what intensity conferred the greatest risk.
METHODS
Overview
We developed a network model to investigate the dynamics of NG transmission among MSM in Belgium. Separable temporal exponential-family random graph models were used to fit and simulate the structure of the sexual partnership network. The model was developed as an extension of the EpiModel platform (www.epimodel.org) and was based on the existing R package EpiModelHIV by Jenness et al [29–31]. The original functions were adapted and extended to account for 3 anatomical sites of gonococcal infection and the Belgian MSM population structure.
The individuals in the population were categorized into high risk and low risk, depending on their risk-taking behaviors. We estimated that 30% of the Belgian MSM population would be eligible to receive preexposure prophylaxis (PrEP), which was used as a proxy for high-risk behavior [32]. Eligibility criteria for PrEP in Belgium are listed in the Supplementary Materials. This estimate is similar to previously published estimates from European studies [33, 34]. The remaining population in the network was classified as low-risk MSM.
Furthermore, the model consisted of 2 interacting networks, representing main and casual partnerships. Each individual in the network had 3 anatomical sites to and from which gonorrhea transmission could occur: pharynx, urethra, and rectum. All definitions, processes, and parameters in the model are described in detail in the Supplementary Materials.
The parameters used to construct the network and inform the processes in the model come from 2 separate sources. Parameters regarding low risk come from a previously published modeling study by Buyze et al, using the Belgium-based participants of the 2010 European MSM Internet Survey (EMIS) [21]. The parameters and code used in this modeling study are open access [21]. Parameters for the high-risk MSM in Belgium were estimated using individual data from the PreGo study [35]. In the PreGo study, a cohort of 343 high-risk MSM were followed up as part of a clinical trial that aimed to assess the efficacy of a mouthwash to prevent STIs.
Partnership Formation and Dissolution
The formation of partnerships was similar for main and casual partnerships and was affected by the total number of active partnerships in the network and the risk group of the individual. The partnership dissolution is modeled as a memoryless process depending on the relationship type and the individual's risk group. The estimates for main and casual partnerships were calibrated to match the number of sex partners reported in the source data.
Sex Acts and Behavioral Characteristics
At each time step, the number of sexual acts that occurred between 2 partners was calculated by random draws from a Poisson distribution with a mean value depending on the type of partnership and the risk group of the 2 partners. Each sex act could be a combination of 6 sex types: oral, oral–anal, and anal sex, each of which could be insertive or receptive. For each sex act, a combination of sex types was randomly selected based on the frequency reported among EMIS 2010 participants as calculated by Buyze et al [21].
Condom use was implemented during anal sex only, as the use of barrier precautions during oral or oral–anal sex is uncommon [36–38]. A proportion of the population was stochastically assigned as consistent condom users with all casual partners, while the rest of the population used condoms on a per-act probability. The same per-act concept was applied to condom use in main partnerships.
Neisseria gonorrhoeae Transmission
Directional transmission of N gonorrhoeae occurred stochastically given the active partnerships, the number of sex acts within a pair, the sex type combinations, the sites involved within each act, and their respective NG infection status, at each time point. The per-act transmission probabilities (same for both risk groups) depended only on the sites involved, the sex role (insertive/receptive) of each partner, and condom use (only for anal sex).
The presence of symptoms in each newly infected site was determined by a random Bernoulli draw, with a different probability for each site. The incubation period of infection was not taken into consideration and infected nodes were considered infectious on the next time step (day). Appearance of symptoms was also assumed to happen immediately. Transmission and symptom probabilities were unavailable in the source data and were calibrated based on information from a literature review (Supplementary Materials).
Treatment and Recovery
A diagnosis of NG could be made based on a positive testing result. Such testing could either be initiated because of symptoms or because an individual attended an STI screening visit. We assumed that 90% of MSM with symptomatic gonorrhea seek testing [30, 39]. Individuals diagnosed with NG were assumed to have a 100% probability to receive dual treatment (ceftriaxone plus azithromycin), according to Belgian and International Union Against Sexually Transmitted Infections–Europe treatment guidelines [13]. We assumed that NG infections could be detected as soon as day 7 after infection. Receiving effective antibiotic treatment was assumed to result in cure from NG across all anatomical sites [40–42]. We evaluated 2 treatment efficacies of dual therapy: 90% and 99%.
Persons diagnosed with gonorrhea were assumed to stochastically cease sexual activity for 7 days in accordance with current Belgian recommendations [13]. Infected but untreated individuals could return to a susceptible state through natural clearance, which was modeled as a stochastic function with different recovery rates for each anatomical site. Expected times for natural clearance were not available in the source data and were calibrated. The expected time to recovery of treated individuals was estimated to be 1 day [43, 44], and equal across all 3 anatomical sites. Upon recovery, individuals were assumed to be susceptible to immediate reinfection [45].
Screening, Treatment, and Antimicrobial Resistance
At each time step, individuals could be randomly selected among all eligible individuals to be screened for NG. The selection depended on the proportion of the risk group we assumed will attend a screening visit and the specified interval between 2 screening visits, which was different for each risk group.
Although there have been no documented cases of high-level ceftriaxone resistance in Belgium, resistance to azithromycin has increased from 0.2% to 18.6% over the past 8 years, despite the use of dual therapy in this period [46]. The increase in azithromycin resistance was most pronounced in MSM, reaching 28% in 2021 versus 14% for heterosexuals [46]. Spontaneous emergence and selection of NG resistance to azithromycin was determined by a stochastic process through a Bernoulli draw with a fixed probability of 1 in 100 000 [47] among all NG-infected individuals who received treatment (and a probability of 1 in 1000 in a sensitivity analysis). Azithromycin-resistant cases of NG could be transmitted to uninfected individuals and to individuals infected with an azithromycin-susceptible NG. Due to the effect of ceftriaxone, azithromycin-resistant cases would still be susceptible to dual therapy with probabilities of eradication of 99% and 90% [48], and recovery would follow the same rules as the azithromycin-susceptible NG cases. Otherwise, the resistant NG-infected anatomical sites would switch to the uninfected state through natural clearance. Once recovered, individuals could be reinfected with NG. Persons who recovered through natural clearance could be reinfected immediately. Among individuals who received dual therapy, the timing until potential reinfection was determined by the differential elimination time of azithromycin (15 days [49, 50]) and ceftriaxone (1 day [51]) from the human body. Thus, reinfection with an azithromycin-resistant NG could occur as soon as 1 day after dual treatment, whereas reinfection with azithromycin-susceptible NG could only happen after 15 days.
Simulation and Calibration
The model simulated a population of 10 000 MSM in Belgium. Parameters for transmission probabilities, symptomatic infection, duration until natural clearance, and probability of abstinence in case of infection were not available in the data sources, so we performed a literature review to identify estimates of other clinical or modeling studies. Approximate Bayesian computation with sequential Monte Carlo sampling was used to estimate the parameters that were not available, using the range of previously published parameters as prior information. The expected median prevalence in the general MSM population (both groups pooled) at the end of 10 years in a scenario without any STI screening was used as a target statistic (8.5% for pharynx, 1.5% for urethral, and 9% for rectal NG [18, 35, 52]).
Analysis and Scenarios
The estimate of 99% treatment efficacy (baseline scenario) was compared with the hypothesized efficacy probability (90%). Twenty-five scenarios of different screening intensities (all possible combinations of 0, 10%, 20%, 30%, and 40% screening between the high- and low-risk MSM) were simulated for each treatment efficacy estimate. Each scenario was simulated 1000 times over a period of 10 years after a burn-in period of 200 days, in daily time steps. Median prevalence and interquartile range over time and at the last day of the 10-year period were the primary endpoints to compare the effect of treatment efficacy. In the case of azithromycin-resistant NG, we opted for the mean period or cumulative prevalence (number of AMR cases over the whole simulation period) with 95% confidence intervals (CIs) instead of the median, due to the very small occurrence probability.
RESULTS
Neisseria gonorrhoeae Prevalence
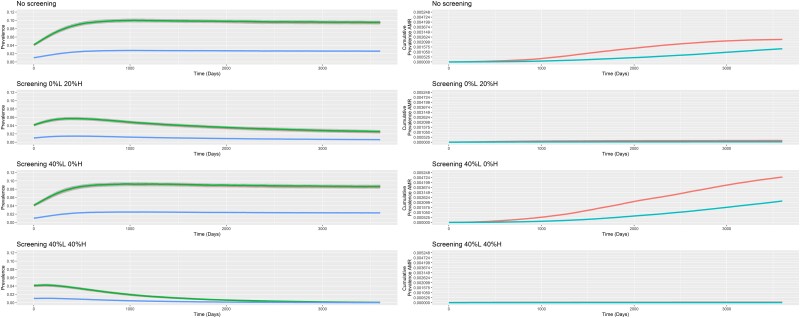
Rectal NG was most prevalent site of infection in all scenarios, followed by pharyngeal and then urethral infections. Figure 1 provides the prevalence by anatomical site over time for 4 indicative screening scenarios where the treatment efficacy is set at 99%. The results for all scenarios and treatment efficacies are presented in the Supplementary Materials. A clear negative association between screening intensity among high-risk MSM and NG prevalence can be observed throughout all scenarios. Increasing screening intensity in the low-risk MSM has little effect on NG prevalence. The prevalence of NG stabilized over time in all scenarios and the different scenarios were compared based on the estimated prevalence at the end of 10 years of simulations (Table 1). Lowering treatment efficacy did not affect the prevalence of NG, producing almost identical figures (Supplementary Figures 18–22).
Figure 1.
Median Neisseria gonorrhoeae (NG) prevalence over time by anatomical site and azithromycin resistance. NG period prevalence over 3600 days by risk group for each screening intensity scenario. Treatment efficacy is set at 99% (green, rectum; red, pharynx; blue, urethra; pink, high-risk men who have sex with men [MSM]; light blue, low-risk MSM). Abbreviations: AMR, antimicrobial resistance; H, high risk; L, low risk.
Table 1.
Median Estimated Neisseria gonorrhoeae Prevalence at the End of 10 Years of Simulations by Screening Intensity Scenario for Treatment Efficacy at 99%
| Screening Scenario | Pharynx, Median (IQR) |
Urethra, Median (IQR) |
Rectum, Median (IQR) |
No. of Antibiotic Treatments per 1000 PD (High Risk) | No. of Antibiotic Treatments per 1000 PD (Low Risk) |
|---|---|---|---|---|---|
| No screening | 9.41 (8.97–9.83) | 2.6 (2.45–2.77) | 9.56 (9.12–9.99) | 2.49 | 0.70 |
| Screening 0%L 10%H | 5.5 (5.03–5.95) | 1.44 (1.31–1.58) | 5.72 (5.2–6.14) | 1.80 | 0.48 |
| Screening 0%L 20%H | 2.45 (2.05–2.81) | 0.62 (0.5–0.73) | 2.57 (2.15–2.95) | 1.21 | 0.30 |
| Screening 0%L 30%H | 0.77 (0.52–1.07) | 0.19 (0.12–0.27) | 0.81 (0.55–1.11) | 0.80 | 0.19 |
| Screening 0%L 40%H | 0.17 (0.05–0.34) | 0.04 (0.01–0.09) | 0.18 (0.06–0.36) | 0.56 | 0.13 |
| Screening 10%L 0%H | 9.11 (8.63–9.55) | 2.5 (2.34–2.68) | 9.25 (8.8–9.74) | 2.44 | 0.70 |
| Screening 10%L 10%H | 5.21 (4.8–5.62) | 1.37 (1.23–1.49) | 5.42 (4.98–5.84) | 1.75 | 0.47 |
| Screening 10%L 20%H | 2.21 (1.82–2.66) | 0.56 (0.45–0.68) | 2.33 (1.93–2.78) | 1.15 | 0.30 |
| Screening 10%L 30%H | 0.66 (0.43–0.94) | 0.17 (0.1–0.23) | 0.69 (0.46–0.99) | 0.76 | 0.19 |
| Screening 10%L 40%H | 0.14 (0.03–0.28) | 0.03 (0–0.07) | 0.14 (0.03–0.3) | 0.54 | 0.13 |
| Screening 20%L 0%H | 8.88 (8.41–9.29) | 2.43 (2.26–2.56) | 9.06 (8.63–9.49) | 2.41 | 0.70 |
| Screening 20%L 10%H | 5.03 (4.53–5.47) | 1.31 (1.16–1.45) | 5.22 (4.75–5.67) | 1.71 | 0.47 |
| Screening 20%L 20%H | 2.03 (1.65–2.41) | 0.5 (0.4–0.61) | 2.12 (1.75–2.55) | 1.12 | 0.29 |
| Screening 20%L 30%H | 0.58 (0.35–0.84) | 0.14 (0.08–0.21) | 0.61 (0.36–0.9) | 0.74 | 0.18 |
| Screening 20%L 40%H | 0.11 (0.02–0.25) | 0.02 (0–0.06) | 0.12 (0.01–0.26) | 0.52 | 0.13 |
| Screening 30%L 0%H | 8.71 (8.25–9.11) | 2.37 (2.21–2.52) | 8.89 (8.46–9.32) | 2.36 | 0.69 |
| Screening 30%L 10%H | 4.78 (4.32–5.2) | 1.22 (1.1–1.36) | 4.98 (4.54–5.43) | 1.67 | 0.46 |
| Screening 30%L 20%H | 1.96 (1.59–2.34) | 0.49 (0.38–0.6) | 2.07 (1.66–2.48) | 1.09 | 0.29 |
| Screening 30%L 30%H | 0.51 (0.31–0.78) | 0.12 (0.07–0.19) | 0.53 (0.32–0.82) | 0.71 | 0.18 |
| Screening 30%L 40%H | 0.1 (0.01–0.23) | 0.02 (0–0.06) | 0.1 (0–0.24) | 0.51 | 0.12 |
| Screening 40%L 0%H | 8.46 (8.04–8.92) | 2.28 (2.13–2.45) | 8.7 (8.27–9.13) | 2.34 | 0.69 |
| Screening 40%L 10%H | 4.7 (4.25–5.09) | 1.2 (1.08–1.33) | 4.89 (4.44–5.32) | 4.91 | 0.36 |
| Screening 40%L 20%H | 1.8 (1.43–2.18) | 0.45 (0.35–0.55) | 1.91 (1.52–2.31) | 3.17 | 0.22 |
| Screening 40%L 30%H | 0.47 (0.26–0.71) | 0.11 (0.06–0.18) | 0.5 (0.28–0.76) | 2.09 | 0.14 |
| Screening 40%L 40%H | 0.06 (0–0.19) | 0.01 (0–0.05) | 0.06 (0–0.2) | 1.49 | 0.09 |
Abbreviations: H, high risk; IQR, interquartile range; L, low risk; PD, person-days.
Antimicrobial-Resistant NG Prevalence
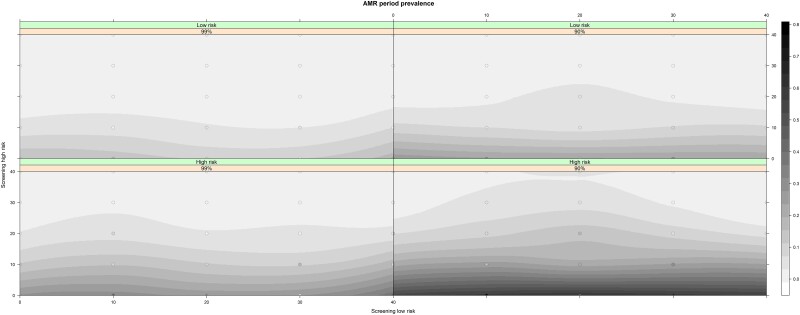
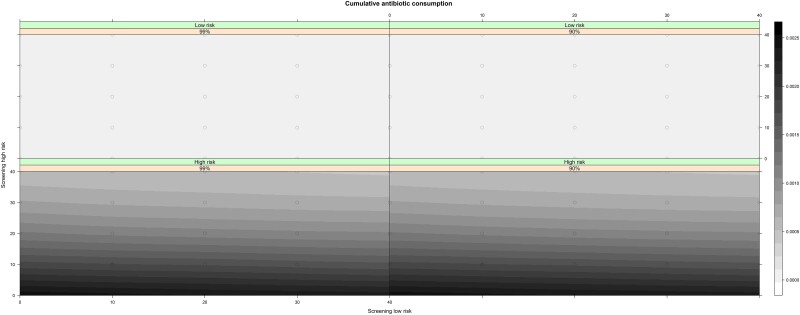
Even though the period prevalence over 3600 days for resistance to azithromycin was higher in the high-risk group compared to the low-risk group for all screening intensity scenarios, it remained low for all scenarios (<0.76% of all NG infections) (Figure 2; Table 2; Supplementary Materials, Section 6). Azithromycin resistance was more prevalent when treatment efficacy was reduced to 90% (Supplementary Table 10). For both treatment efficacy scenarios, increasing screening in the high-risk population decreased both the NG prevalence and the risk for azithromycin resistance (Figure 2) irrespective of the screening intensity in the low-risk group. Nevertheless, high-risk MSM were at higher risk for azithromycin-resistant NG in all screening intensity and treatment efficacy scenarios (Figure 1; Supplementary Materials, Section 6.1). Overall, irrespective of treatment efficacy, intensive screening in the low-risk group without screening high-risk MSM resulted in the highest cumulative antibiotic consumption (Table 1; Figure 3).
Figure 2.
Azithromycin-resistant Neisseria gonorrhoeae period prevalence over 3600 days by screening intensity and treatment efficacy. Actual results from the screening combinations are depicted with a circle. Abbreviation: AMR, antimicrobial resistance.
Table 2.
Mean Azithromycin-Resistant Neisseria gonorrhoeae Period Prevalence at the End of 10 Years of Simulations by Screening Intensity and Treatment Efficacy for the Overall Men Who Have Sex With Men Population and by Risk Group
| Period Prevalence | Treatment Efficacy | |||||
|---|---|---|---|---|---|---|
| Overall, % (95% CI) |
High Risk, % (95% CI) |
Low Risk, % (95% CI) |
||||
| 99% | 90% | 99% | 90% | 99% | 90% | |
| No screening | 0.17 (.1–0.28) | 0.33 (.23–0.47) | 0.24 (.1–.53) | 0.48 (.26–.84) | 0.14 (.07–.28) | 0.26 (.16–.43) |
| Screening 0%L 10%H | 0.07 (.03–.16) | 0.1 (.05–.19) | 0.15 (.05–.41) | 0.2 (.07–.48) | 0.04 (.01–.14) | 0.05 (.01–.16) |
| Screening 0%L 20%H | 0.01 (0–.07) | 0.05 (.02–.14) | 0.02 (0–.22) | 0.11 (.03–.36) | 0 (0–.09) | 0.03 (0–.12) |
| Screening 0%L 30%H | 0 (0–.06) | 0.01 (0–.07) | 0.01 (0–.2) | 0.02 (0–.22) | 0 (0–.08) | 0 (0–.09) |
| Screening 0%L 40%H | 0 (0–.06) | 0 (0–.06) | 0.01 (0–.2) | 0.01 (0–.2) | 0 (0–.08) | 0 (0–.08) |
| Screening 10%L 0%H | 0.25 (.16–.38) | 0.42 (.3–.58) | 0.4 (.21–.74) | 0.76 (.48–1.18) | 0.19 (.1–.34) | 0.28 (.17–.45) |
| Screening 10%L 10%H | 0.05 (.02–.13) | 0.09 (.04–.18) | 0.09 (.02–.33) | 0.19 (.07–.47) | 0.03 (0–.13) | 0.05 (.01–.15) |
| Screening 10%L 20%H | 0.05 (.02–.13) | 0.04 (.01–.11) | 0.1 (.02–.34) | 0.08 (.01–.31) | 0.03 (0–.12) | 0.02 (0–.11) |
| Screening 10%L 30%H | 0.01 (0–.06) | 0.03 (.01–.1) | 0.01 (0–.2) | 0.06 (.01–.29) | 0 (0–.08) | 0.02 (0–.11) |
| Screening 10%L 40%H | 0.01 (0–.07) | 0.01 (0–.07) | 0.02 (0–.23) | 0.02 (0–.21) | 0.01 (0–.09) | 0 (0–.09) |
| Screening 20%L 0%H | 0.16 (.09–.27) | 0.27 (.18–.4) | 0.28 (.12–.58) | 0.47 (.26–.83) | 0.1 (.04–.23) | 0.18 (.1–.33) |
| Screening 20%L 10%H | 0.06 (.02–.14) | 0.11 (.05–.21) | 0.11 (.03–.36) | 0.22 (.08–.51) | 0.03 (.01–.13) | 0.06 (.02–.17) |
| Screening 20%L 20%H | 0.01 (0–.07) | 0.1 (.05–.2) | 0.02 (0–.23) | 0.19 (.07–.46) | 0.01 (0–.09) | 0.06 (.02–.17) |
| Screening 20%L 30%H | 0.01 (0–.07) | 0.02 (0–.08) | 0.02 (0–.21) | 0.04 (0–.24) | 0 (0–.09) | 0.01 (0–.09) |
| Screening 20%L 40%H | 0 (0–.06) | 0.02 (0–.08) | 0 (0–.19) | 0.04 (0–.25) | 0 (0–.08) | 0.01 (0–.09) |
| Screening 30%L 0%H | 0.05 (.02–.13) | 0.38 (.27–.53) | 0.11 (.03–.36) | 0.61 (.37–1) | 0.03 (0–.12) | 0.28 (.17–.45) |
| Screening 30%L 10%H | 0.09 (.04–.18) | 0.15 (.08–.25) | 0.17 (.06–.44) | 0.29 (.13–.61) | 0.06 (.01–.16) | 0.08 (.03–.2) |
| Screening 30%L 20%H | 0.01 (0–.07) | 0.03 (.01–.1) | 0.02 (0–.22) | 0.06 (.01–.29) | 0 (0–.09) | 0.02 (0–.11) |
| Screening 30%L 30%H | 0.01 (0–.07) | 0.01 (0–.07) | 0.02 (0–.22) | 0.02 (0–.21) | 0 (0–.09) | 0 (0–.09) |
| Screening 30%L 40%H | 0 (0–.06) | 0.01 (0–.07) | 0.01 (0–.2) | 0.02 (0–.23) | 0 (0–.08) | 0.01 (0–.09) |
| Screening 40%L 0%H | 0.3 (.2–.44) | 0.33 (.22–.47) | 0.48 (.26–.84) | 0.57 (.33–.95) | 0.23 (.13–.38) | 0.22 (.13–.38) |
| Screening 40%L 10%H | 0.04 (.01–.12) | 0.1 (.05–.19) | 0.09 (.01–.32) | 0.19 (.07–.46) | 0.02 (0–.11) | 0.06 (.02–.17) |
| Screening 40%L 20%H | 0.03 (.01–.1) | 0.03 (0–.1) | 0.06 (.01–.28) | 0.05 (0–.27) | 0.02 (0–.11) | 0.01 (0–.1) |
| Screening 40%L 30%H | 0 (0–.06) | 0.02 (0–.08) | 0.01 (0–.2) | 0.03 (0–.24) | 0 (0–.08) | 0.01 (0–.09) |
| Screening 40%L 40%H | 0 (0–.06) | 0.01 (0–.06) | 0 (0–.19) | 0.01 (0–.2) | 0 (0–.08) | 0 (0–.08) |
Abbreviations: CI, confidence interval; H, high risk; L, low risk.
Figure 3.
Cumulative number of antibiotic treatments administered in the population over 10 years for each screening intensity scenario and treatment efficacy.
Sensitivity Analysis
A sensitivity analysis with an increased probability of azithromycin-resistant NG emergence at 1/1000 was performed and resulted in more realistic figures (overall prevalence, 10.43% [95% CI, 9.8%–11.09%]). All results from the sensitivity analysis are presented in the Supplementary Materials.
DISCUSSION
Our model examined the impact of screening on NG prevalence and the emergence of gonococcal resistance to azithromycin in an MSM population. In all 3 anatomical sites, and in keeping with previous studies [22, 23, 25], we found a dose-response effect between increased screening intensity and reduced NG prevalence. Elimination of NG was not, however, observed in any scenario evaluated. High-risk MSM appear to play a critical role in the transmission of NG in MSM, since any levels of screening in low-risk MSM had little effect on NG prevalence.
Our model suggests that the period prevalence of resistance to azithromycin was higher in the high-risk MSM in all scenarios where resistance emerged, compared to the low-risk group. This finding is in keeping with the frequent emergence of gonococcal AMR in core groups [53]. A plausible mechanism for this finding is that the dense sexual network of this population generates a high equilibrium prevalence of NG, which places it at a high probability for AMR emerging if exposed to high levels of antimicrobials [53]. The highest levels of azithromycin resistance were observed in scenarios where no screening was conducted in the high-risk group regardless of screening intensity in the low-risk group. Even in the most screening-intensive scenario for the low-risk group (annual screening in 40% of the group), screening had little impact on the prevalence of NG or azithromycin resistance. In contrast, screening in high-risk MSM had a large impact, even at low intensity levels. A possible explanation for these findings is that screening the more active individuals in the high-risk population reduces the prevalence of NG over time to such an extent that it reduces the cumulative number of NG infections (symptomatic or asymptomatic) requiring antibiotic therapy. Screening among the low-risk MSM, however, reduces the prevalence of NG only slightly, meaning that the residual symptomatic NG cases plus the antimicrobials used in screening result in an increased probability of AMR emerging.
Our results thus suggest that intensive screening in the low-risk population has little impact on prevalence but may increase the probability of AMR emerging via increasing antimicrobial consumption. In contrast, intensive screening in the high-risk population reduces both the prevalence of NG and macrolide resistance.
Unsurprisingly, we found that the risk of AMR emerging was highest when the treatment efficacy decreased. While it may be objected that a prevalence of 10% combined resistance to ceftriaxone and azithromycin (90% treatment efficacy scenarios) is unrealistic, a recent study from China has reported combined ceftriaxone/azithromycin resistance/reduced susceptibility prevalence of 9.3% [54]. Our findings suggest that the optimal screening intensity may vary according to local prevalence of resistance to the antimicrobials used for treating NG.
The prevalence of gonococcal resistance to azithromycin that emerged in all scenarios in our model was considerably lower than the actual prevalence of azithromycin observed in Belgian MSM over the past 8 years, which increased from 0.2% in 2013 to 28% in 2021 [46]. This may be explained by our 1/100 000 probability of azithromycin resistance emerging being too low or by the fact that we did not incorporate the option of AMR emerging via horizontal gene transfer from commensal Neisseria species or bystander selection [55]. Bystander selection refers to the process whereby an antibiotic given to treat 1 infection induces AMR in other bacteria that are resident at the time of receipt of the antibiotic [56]. Bystander selection has been estimated to drive 5%–98% of AMR in NG [56]. The use of macrolides to treat other STIs such as chlamydia and Mycoplasma genitalium is an important determinant of total macrolide consumption in MSM [16]. Horizontal transfer of resistance conferring DNA from commensal Neisseria species to NG was shown to have played an important role in the genesis of gonococcal resistance to macrolides and other antimicrobials [57, 58]. Because the prevalence of commensal Neisseria is close to 100%, these bacteria are particularly affected by bystander selection [59]. We plan to incorporate bystander selection and horizontal gene transfer from commensal Neisseria in future iterations of this model.
Our model has a number of other limitations. First, treatment was assumed to be uniform across all 3 anatomical sites of infection, disregarding poorer azithromycin penetration into the oropharynx, which could lower the efficacy of treatment against NG. Second, we lacked accurate estimates of a number of the model parameters such as transmission efficacy and duration of infection, as well as accurate behavioral data by risk group. We did not explicitly include an incubation period of NG in the model; however, we did specify a delay of 7 days between infection and the diagnosis of NG, to account for the incubation period and diagnostic delays. The structure of the model, although allowing for fine-grained description of the population, still deviates from reality due to practical limitations, mainly in the form of lack of good-quality data. Our model does not include the age of individuals or any temporal changes, either in the consistency of the population (no entries or exits from the network) or in the behavioral characteristics of individuals (people cannot change risk group over time). Despite the many limitations, our study also has significant strengths, as it attempts to explain the complex mechanism of NG transmission and AMR emergence in a highly detailed network model. Previously published models either used a compartmental approach, did not include details on all 3 anatomical sites, or did not include antibiotic intake and the emergence of antimicrobial-resistant NG in the population [21–23, 26, 27, 30]. Future models that are better able to capture the pathways whereby NG acquires AMR will hopefully more precisely delineate the optimal NG screening intensity in MSM that has the maximal effect on lowering NG prevalence and the least risk of inducing AMR.
Supplementary Material
Contributor Information
Achilleas Tsoumanis, Department of Clinical Sciences, Institute of Tropical Medicine Antwerp, Antwerp, Belgium; Centre for Health Economics Research and Modelling Infectious Diseases, Vaccine and Infectious Disease Institute, University of Antwerp, Antwerp, Belgium.
Christophe Van Dijck, Department of Clinical Sciences, Institute of Tropical Medicine Antwerp, Antwerp, Belgium; Laboratory of Medical Microbiology, University of Antwerp, Antwerp, Belgium.
Niel Hens, Centre for Health Economics Research and Modelling Infectious Diseases, Vaccine and Infectious Disease Institute, University of Antwerp, Antwerp, Belgium; Interuniversity Institute for Biostatistics and Statistical Bioinformatics, Data Science Institute, Hasselt University, Hasselt, Belgium.
Chris Kenyon, Department of Clinical Sciences, Institute of Tropical Medicine Antwerp, Antwerp, Belgium; Division of Infectious Diseases and HIV Medicine, Department of Medicine, University of Cape Town, Cape Town, South Africa.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank Jozefien Buyze for the insights into the initial conception of the model and Lander Willem for critical assistance during the model calibration.
Patient consent. The parameters in this study are derived from already published papers. In the case of primary data from the PreGo study, written informed consent was obtained from all participants. The PreGo study obtained an ethics approval from the Institutional Review Board of the Institute of Tropical Medicine (1276/18) and from the Ethics Committee of the University of Antwerp (19/06/058).
References
- 1. European Centre for Disease Prevention and Control (ECDC) . Gonorrhoea: annual epidemiological report for 2018. 2020. Available at: https://www.ecdc.europa.eu/sites/default/files/documents/gonorrhoea-annual-epidemiological-report-2018.pdf. Accessed July 20, 2022.
- 2. Centers for Disease Prevention and Control (CDC) . Sexually transmitted disease surveillance 2019. Atlanta, GA: CDC; 2021.
- 3. World Health Organization (WHO) . Prevention and treatment of HIV and other sexually transmitted infections among men who have sex with men and transgender people. Recommendations for a public health approach.2011. Available at: http://apps.who.int/iris/bitstream/10665/44619/1/9789241501750_eng.pdf. Accessed May 29, 2022.
- 4. Workowski KA, Bolan GA; Centers for Disease Control and Prevention . Sexually transmitted diseases treatment guidelines. MMWR Recomm Rep 2015; 64(RR-03):1–137. [PMC free article] [PubMed] [Google Scholar]
- 5. Asboe D, Aitken C, Boffito M, et al. British HIV Association guidelines for the routine investigation and monitoring of adult HIV-1-infected individuals 2011. HIV Med 2012; 13:1–44. [DOI] [PubMed] [Google Scholar]
- 6. Sexually Transmissible Infections in Gay Men Action Group . Australian sexually transmitted infection and HIV testing guidelines 2014 for asymptomatic men who have sex with men. 2014. Available at: http://stipu.nsw.gov.au/wp-content/uploads/STIGMA_Testing_Guidelines_Final_v5.pdf. Accessed May 29, 2022.
- 7. Ingleton A, Hope K, Najjar Z, Templeton DJ, Gupta L. Characteristics of gonorrhoea cases notified in inner and south-western Sydney, Australia: results of population-based enhanced surveillance. Sex Health 2016; 13:484–8. [DOI] [PubMed] [Google Scholar]
- 8. Public Health Agency of Canada . Canadian guidelines on sexually transmitted infections. Ottawa: Public Health Agency of Canada; 2010.
- 9. European Centre for Disease Prevention and Control (ECDC) . HIV and STI prevention among men who have sex with men. Stockholm, Sweden: ECDC; 2015. [Google Scholar]
- 10. Low N, Unemo M, Skov Jensen J, Breuer J, Stephenson JM. Molecular diagnostics for gonorrhoea: implications for antimicrobial resistance and the threat of untreatable gonorrhoea. PLoS Med 2014; 11:e1001598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kirkcaldy RD, Harvey A, Papp JR, et al. Antimicrobial susceptibility surveillance—the gonococcal isolate surveillance project, 27 sites, United States, 2014. MMWR Surveill Summ 2016; 65:1–19. [DOI] [PubMed] [Google Scholar]
- 12. Centers for Disease Control and Prevention . STD Prevention Conference: new warning signs that gonorrhea treatment may be losing effectiveness [press release]. 2016. Available at: http://www.cdc.gov/nchhstp/newsroom/2016/2016-std-prevention-conference-press-release.html. Accessed 30 June 2017.
- 13. Jespers V, Stordeur S, Desomer A, et al. Sexually transmitted infections in primary care consultations: development of an online tool to guide healthcare practitioners. 2019. Available at: www.kce.fgov.be.
- 14. World Health Organization (WHO) . WHO guidelines for the treatment of Neisseria gonorrhoeae. Geneva, Switzerland: WHO; 2016.
- 15. Vanbaelen T, Van Dijck C, De Baetselier I, et al. Screening for STIs is one of the main drivers of macrolide consumption in PrEP users. Int J STD AIDS 2021; 32:1183–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kenyon C, De Baetselier I, Wouters K. Screening for STIs in PrEP cohorts results in high levels of antimicrobial consumption. Int J STD AIDS 2020; 31:1215–8. [DOI] [PubMed] [Google Scholar]
- 17. Kenyon C. Dual azithromycin/ceftriaxone therapy for gonorrhea in PrEP cohorts results in levels of macrolide consumption that exceed resistance thresholds by up to 7-fold. J Infect Dis 2021; 224:1623–4. [DOI] [PubMed] [Google Scholar]
- 18. Tsoumanis A, Hens N, Kenyon CR. Is screening for chlamydia and gonorrhea in men who have sex with men associated with reduction of the prevalence of these infections? A systematic review of observational studies. Sex Transm Dis 2018; 45:615–22. [DOI] [PubMed] [Google Scholar]
- 19. Marcus U, Mirandola M, Schink SB, Gios L, Schmidt AJ. Changes in the prevalence of self-reported sexually transmitted bacterial infections from 2010 and 2017 in two large European samples of men having sex with men—is it time to reevaluate STI-screening as a control strategy? PLoS One 2021; 16:e0248582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kenyon C. Screening is not associated with reduced incidence of gonorrhoea or chlamydia in men who have sex with men (MSM); an ecological study of 23 European countries. F1000Res 2019; 8:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Buyze J, Vanden Berghe W, Hens N, Kenyon C. Current levels of gonorrhoea screening in MSM in Belgium may have little effect on prevalence: a modelling study. Epidemiol Infect 2018; 146:333–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Earnest R, Rönn MM, Bellerose M, et al. Population-level benefits of extragenital gonorrhea screening among men who have sex with men: an exploratory modeling analysis. Sex Transm Dis 2020; 47:484–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kretzschmar M, Van Duynhoven YTHP, Severijnen AJ. Modeling prevention strategies for gonorrhea and chlamydia using stochastic network simulations. Am J Epidemiol 1996; 144:306–17. [DOI] [PubMed] [Google Scholar]
- 24. Garnett GP, Mertz KJ, Finelli L, Levine WC, Louis ME. The transmission dynamics of gonorrhoea: modelling the reported behaviour of infected patients from Newark, New Jersey. Philos Trans R Soc B Biol Sci 1999; 354:787–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tuite AR, Rönn MM, Wolf EE, et al. Estimated impact of screening on gonorrhea epidemiology in the United States: insights from a mathematical model. Sex Transm Dis 2018; 45:713–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tuite AR, Gift TL, Chesson HW, Hsu K, St Salomon JA, Grad YH. Impact of rapid susceptibility testing and antibiotic selection strategy on the emergence and spread of antibiotic resistance in gonorrhea. J Infect Dis 2017; 216:1141–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yaesoubi R, Cohen T, Hsu K, et al. Adaptive guidelines for the treatment of gonorrhea to increase the effective life span of antibiotics among men who have sex with men in the United States: a mathematical modeling study. PLoS Med 2020; 17:e1003077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hui B, Fairley CK, Chen M, et al. Oral and anal sex are key to sustaining gonorrhoea at endemic levels in MSM populations: a mathematical model. Sex Transm Infect 2015; 91:365–9. [DOI] [PubMed] [Google Scholar]
- 29. Goodreau SM, Carnegie NB, Vittinghoff E, et al. What drives the US and Peruvian HIV epidemics in men who have sex with men (MSM)? PLoS One 2012; 7:e50522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jenness SM, Weiss KM, Goodreau SM, et al. Incidence of gonorrhea and chlamydia following human immunodeficiency virus preexposure prophylaxis among men who have sex with men: a modeling study. Clin Infect Dis 2017; 65:712–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jenness SM, Goodreau SM, Rosenberg E, et al. Impact of the Centers for Disease Control's HIV preexposure prophylaxis guidelines for men who have sex with men in the United States. J Infect Dis 2016; 214:1800–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Buffel V, Reyniers T, Masquillier C, et al. Awareness of, willingness to take PrEP and its actual use among Belgian msm at high risk of HIV infection: secondary analysis of the Belgian European MSM internet survey. AIDS Behav 2022; 26:1793–807. [DOI] [PubMed] [Google Scholar]
- 33. Sherriff NS, Jones AM, Mirandola M, et al. Factors related to condomless anal intercourse between men who have sex with men: results from a European bio-behavioural survey. J Public Health (Oxf) 2020; 42:e174–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hansson D, Strömdahl S, Leung KY, Britton T. Introducing pre-exposure prophylaxis to prevent HIV acquisition among men who have sex with men in Sweden: insights from a mathematical pair formation model. BMJ Open 2020; 10:e033852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Van Dijck C, Tsoumanis A, Rotsaert A, et al. Antibacterial mouthwash to prevent sexually transmitted infections in men who have sex with men taking HIV pre-exposure prophylaxis (PReGo): a randomised, placebo-controlled, crossover trial. Lancet Infect Dis 2021; 21:657–67. [DOI] [PubMed] [Google Scholar]
- 36. Glynn T, Operario D, Montgomery M, Almonte A, Chan P. The duality of oral sex for men who have sex with men: an examination into the increase of sexually transmitted infections amid the age of HIV prevention. AIDS Patient Care STDS 2017; 31:261–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Giano Z, Kavanaugh KE, Durham AR, et al. Factors associated with condom use among a sample of men who have sex with men (MSM) residing in rural Oklahoma. J Homosex 2020; 67:1881–901. [DOI] [PubMed] [Google Scholar]
- 38. van den Boom W, Stolte IG, Roggen A, Theo S, Prins M, Davidovich U. Is anyone around me using condoms? Site-specific condom-use norms and their potential impact on condomless sex across various gay venues and websites in the Netherlands. Heal Psychol 2015; 34:857–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Farley TA, Cohen DA, Elkins W. Asymptomatic sexually transmitted diseases: the case for screening. Prev Med 2003; 36:502–9. [DOI] [PubMed] [Google Scholar]
- 40. Kong FYS, Hatzis CL, Lau A, et al. Treatment efficacy for pharyngeal Neisseria gonorrhoeae: a systematic review and meta-analysis of randomized controlled trials. J Antimicrob Chemother 2020; 75:3109–19. [DOI] [PubMed] [Google Scholar]
- 41. Moran J. Treating uncomplicated Neisseria gonorrhoeae infections. Sex Transm Dis 1995; 22:39–47. [DOI] [PubMed] [Google Scholar]
- 42. Yang J, Dhital S, Naderer T. Efficacy and safety of injectable and oral antibiotics in treating gonorrhea: a systematic review and network meta-analysis. J Clin Med 2019; 8:2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zajdowicz TR, Sanches PL, Berg SW, Kerbs SB, Newquist RL, Harrison WO. Comparison of ceftriaxone with cefoxitin in the treatment of penicillin-resistant gonococcal urethritis. Sex Transm Infect 1983; 59:176–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Haizlip J, Isbey SF, Hamilton HA, et al. Time required for elimination of neisseria gonorrhoeae from the urogenital tract in men with symptomatic urethritis. Sex Transm Dis 1995; 22:145–8. [DOI] [PubMed] [Google Scholar]
- 45. Liu Y, Feinen B, Russell MW. New concepts in immunity to Neisseria gonorrhoeae: innate responses and suppression of adaptive immunity favor the pathogen, not the host. Front Microbiol 2011; 2:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. De Baetselier I, Cuylaerts V, Smet H, et al. Neisseria gonorrhoeae antimicrobial resistance surveillance report of Belgium—2021. 2022. Available at: https://nrchm.wiv-isp.be/nl/ref_centra_labo/sti_treponema_pallidum/Rapporten/Neisseriagonorrhoeae 2021.pdf. Accessed December 15, 2022.
- 47. Chan CH, McCabe CJ, Fisman DN. Core groups, antimicrobial resistance and rebound in gonorrhoea in North America. Sex Transm Infect 2012; 88:200–4. [DOI] [PubMed] [Google Scholar]
- 48. Unemo M, Ross JDC, Serwin AB, Gomberg M, Cusini M, Jensen JS. Background review for the 2020 European guideline for the diagnosis and treatment of gonorrhoea in adults. Int J STD AIDS 2021; 32:108–26. [DOI] [PubMed] [Google Scholar]
- 49. Singlas E. Clinical pharmacokinetics of azithromycin [in French]. Pathol Biol (Paris) 1995; 43:505–11. [PubMed] [Google Scholar]
- 50. Di Paolo A, Barbara C, Chella A, Angeletti CA, Del Tacca M. Pharmacokinetics of azithromycin in lung tissue, bronchial washing, and plasma in patients given multiple oral doses of 500 and 1000 mg daily. Pharmacol Res 2002; 46:545–50. [DOI] [PubMed] [Google Scholar]
- 51. Patel IH, Kaplan SA. Pharmacokinetic profile of ceftriaxone in man. Am J Med 1984; 77:17–25. [PubMed] [Google Scholar]
- 52. Vuylsteke B, Reyniers T, De Baetselier I, et al. Daily and event-driven pre-exposure prophylaxis for men who have sex with men in Belgium: results of a prospective cohort measuring adherence, sexual behaviour and STI incidence. J Int AIDS Soc 2019; 22:e25407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kenyon CR, Schwartz IS. Effects of sexual network connectivity and antimicrobial drug use on antimicrobial resistance in Neisseria gonorrhoeae. Emerg Infect Dis 2018; 24:1195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lin X, Qin X, Wu X, et al. Markedly increasing antibiotic resistance and dual treatment of Neisseria gonorrhoeae isolates in Guangdong, China, from 2013 to 2020. Antimicrob Agents Chemother 2022; 66:e0229421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kenyon C, Laumen J, Manoharan-Basil S. Choosing new therapies for gonorrhoea: we need to consider the impact on the pan-Neisseria genome. a viewpoint. Antibiotics 2021; 10:515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tedijanto C, Olesen SW, Grad YH, Lipsitch M. Estimating the proportion of bystander selection for antibiotic resistance among potentially pathogenic bacterial flora. Proc Natl Acad Sci U S A 2018; 115:E11988–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sánchez-Busó L, Cole MJ, Spiteri G, et al. Europe-wide expansion and eradication of multidrug-resistant Neisseria gonorrhoeae lineages: a genomic surveillance study. Lancet Microbe 2022; 3:e452–63. [DOI] [PubMed] [Google Scholar]
- 58. Banhart S, Selb R, Oehlmann S, et al. The mosaic mtr locus as major genetic determinant of azithromycin resistance of Neisseria gonorrhoeae—Germany, 2018. J Infect Dis 2021; 224:1398–404. [DOI] [PubMed] [Google Scholar]
- 59. Laumen JGE, Van Dijck C, Abdellati S, et al. Antimicrobial susceptibility of commensal Neisseria in a general population and men who have sex with men in Belgium. Sci Rep 2022; 12:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.