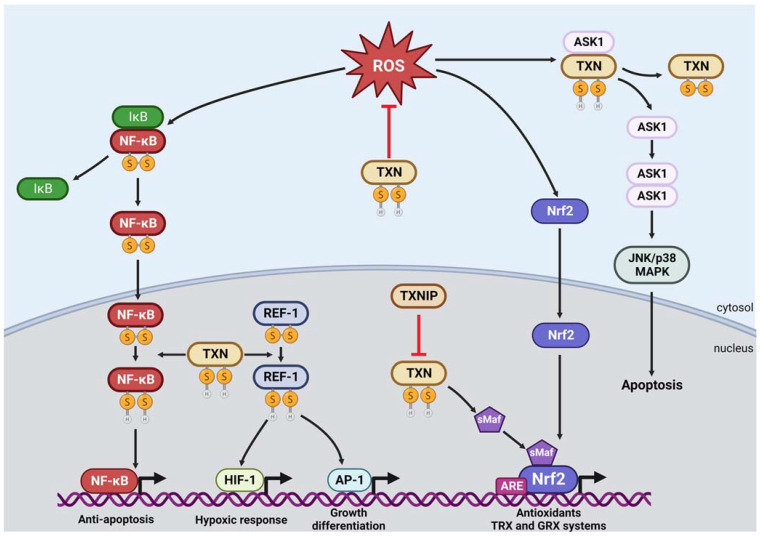
Figure 4.
Role of thioredoxin in redox signaling. Thioredoxin (TXN) negatively regulates apoptosis via redox regulation of ASK-1 and inhibition of Iκβ degradation by scavenging ROS in the cytoplasm. In the nucleus, TXN increases the DNA-binding activity of NF-κβ and can enhance the binding of Nrf2 to the antioxidant response element (ARE) through small Maf proteins (sMaf) via reduction of their cysteine residues. TXN also increases the DNA-binding activity of other transcription factors, such as AP-1 and HIF-1, indirectly via the reduction of intermediate Ref-1 cysteine residues. Thioredoxin-interacting protein (TXNIP) can inhibit TXN function by forming a mixed disulfide bond with its reduced form. Note that although thioredoxin reductase is not depicted in this figure, it is important for the reduction of thioredoxin into its reduced, active form. It is the reduced, active form of thioredoxin that contributes to redox signaling. Red lines indicate an inhibitory effect. S-S = oxidized form. SH = reduced form.