Abstract
Background and Objectives
Sex hormones may modulate calcitonin gene-related peptide (CGRP) release in the trigeminovascular system. We studied CGRP concentrations in plasma and tear fluid in female participants with episodic migraine (EM) and a regular menstrual cycle (RMC), female participants with EM and combined oral contraception (COC), and female participants with EM in the postmenopause. For control, we analyzed 3 corresponding groups of age-matched female participants without EM.
Methods
Participants with an RMC had 2 visits: during menstruation on menstrual cycle day 2 ± 2 and in the periovulatory period on day 13 ± 2. Participants with COC were examined at day 4 ± 2 of the hormone-free interval (HFI) and between days 7 and 14 of hormone intake (HI). Postmenopausal participants were assessed once at a random time point. Plasma and tear fluid samples were collected at each visit for determination of CGRP levels with an ELISA.
Results
A total of 180 female participants (n = 30 per group) completed the study. Participants with migraine and an RMC showed statistically significantly higher CGRP concentrations in plasma and tear fluid during menstruation compared with female participants without migraine (plasma: 5.95 pg/mL [IQR 4.37–10.44] vs 4.61 pg/mL [IQR 2.83–6.92], p = 0.020 [Mann-Whitney U test]; tear fluid: 1.20 ng/mL [IQR 0.36–2.52] vs 0.4 ng/mL [IQR 0.14–1.22], p = 0.005 [Mann-Whitney U test]). In contrast, female participants with COC and in the postmenopause had similar CGRP levels in the migraine and the control groups. In migraine participants with an RMC, tear fluid but not plasma CGRP concentrations during menstruation were statistically significantly higher compared with migraine participants under COC (p = 0.015 vs HFI and p = 0.029 vs HI, Mann-Whitney U test).
Discussion
Different sex hormone profiles may influence CGRP concentrations in people, with current or past capacity to menstruate, with migraine. Measurement of CGRP in tear fluid was feasible and warrants further investigation.
The prevalence of migraine is 3 times higher in women than in men.1 Fluctuations of sex hormones play a crucial role in the pathophysiology of the disease.2 The estrogen withdrawal hypothesis suggests that a drop in estrogen plasma concentrations can trigger migraine attacks.3 In line with this hypothesis, migraine frequency and pain severity are higher during the perimenstrual phase of the menstrual cycle but also in the perimenopausal period before hormonal stabilization at an older age.2,4 Migraine prevalence gradually declines after natural menopause.5
Hormonal contraception leads to the suppression of physiologic hormonal fluctuations with variable effects on migraine.6 The most common hormonal contraception in Europe and North America are combined estrogen-progesterone oral compounds (combined oral contraceptives [COCs]).7 Although some patients experience an improvement of migraine with COC, others experience worsening, with migraine attacks occurring most frequently during the 7-day hormone-free interval (HFI).6
The pathophysiologic mechanisms leading from hormonal changes to the development of migraine attacks are complex. The neuropeptide calcitonin gene-related peptide (CGRP) has a key role in migraine initiation8 and is likely to have a relevant function in the processes initiated by sex hormone changes. During a migraine attack, CGRP is released from trigeminal afferents and triggers an inflammatory response.9 Preclinical research suggests that sex hormone fluctuations can lead to activation of the trigeminovascular system and subsequent release of CGRP, which may contribute to the high prevalence of migraine in female persons of childbearing age.10 However, the clinical evidence in humans is inconclusive. Although older investigations suggest a direct relationship between estrogen and CGRP concentrations,11,12 newer studies imply a higher CGRP release in low estrogen phases.13,14
The accurate measurement of CGRP in peripheral blood is challenging due to its very short half-life time, degradation, and dilution effects after release.15 A recent pilot study detected increased CGRP concentrations in tear fluid in participants with migraine compared with control participants without migraine.16 This exploratory method is noninvasive and could provide a more direct measurement of the trigeminal CGRP release due to its spatial proximity to the trigeminal nerve.
Here, we studied CGRP concentrations in both plasma and tear fluid of female participants with migraine and female participants without migraine under different hormonal conditions. We aimed to assess the relationship between sex hormones and CGRP levels and whether the presence of migraine affects this relationship. It was our hypothesis that (1) female persons with migraine display higher CGRP concentrations than female persons without migraine during the physiologic menstrual cycle and (2) that the suppression of naturally occurring sex hormones through COC or after menopause is associated with changes in the CGRP concentrations.
Methods
Study Design and Participants
This is a cross-sectional, matched-cohort study at the Headache Center, Department of Neurology, Charité Universitätsmedizin Berlin, Berlin, Germany. The study cohort consisted of 3 groups of female participants with episodic migraine (EM): (1) with a regular menstrual cycle (M-RMC); (2) under contraceptive treatment with a COC (M-COC); and (3) during the postmenopause (M-PM). For control, we studied 3 respective groups of age-matched control female participants without EM (C-RMC, C-COC, and C-PM).
Participants with migraine were recruited from our outpatient headache clinic. For the recruitment of participants without migraine, we contacted hospital and university staff via announcements in mailing lists or direct approach.
Inclusion and Exclusion Criteria
EM was defined according to the International Classification of Headache Disorders 3.17 All female participants with migraine should have had at least 3 days with migraine in the 4 weeks before screening, as documented in a headache diary.
An RMC was defined as the cycle duration of 28 ± 2 days in the 3 months before screening. In this group, the diagnosis of menstrually related migraine17 was required for study participation. For inclusion in the COC groups, female participants should confirm the regular use of the same contraceptive drug in a 21/7 regimen (i.e., 21 days of hormone intake [HI] followed by a 7-day HFI), beginning at least 3 months before screening. For the postmenopausal groups, the last menstruation should have occurred at least 5 years before inclusion in the study.
Exclusion criteria were any other diagnosed primary headache disorder except tension‐type headache on less than 2 days in the month before screening; concurrent migraine preventive drug treatment; any gynecologic or other neurologic diseases; ophthalmologic conditions interfering with lacrimation; any other relevant diseases requiring regular medication; hormonal treatment with indications other than contraception; pregnancy; lactation; and poststerilization. For participants with migraine and an RMC, the diagnosis of pure menstrual migraine17 led to exclusion from the study.
Study Procedures
Before the beginning of experimental procedures, potential participants were screened for eligibility. Eligible individuals had an initial interview to record their medical history and a physical examination. In participants with migraine, we reviewed their headache calendars of the month before screening.
The study protocol for female participants with an RMC consisted of 2 study visits. The first visit was scheduled at day 2 ± 2 of the menstrual cycle (during menstruation), whereas the second visit took place at day 13 ± 2 of the menstrual cycle (periovulatory period). These time intervals were selected because estrogen levels are at their lowest during menstruation and at their highest during ovulation.
Female participants with COC were assessed twice: at day 4 ± 2 of the HFI and between days 7–14 of HI. Postmenopausal female participants had only 1 visit at a variable time point.
All visits in participants with migraine were performed in the interictal period, defined as a state free of any migraine symptoms and free of acute pain medication for 12 hours before and after each visit. Participants were instructed to call and reschedule the appointment in case of migraine or acute medication intake within 12 hours before the scheduled visit. We also contacted all participants by phone the day after each visit and asked about any migraine symptoms or medication intake in the 12 hours after the study visit. If this was the case, the visit was repeated at the next possible time point.
Sample Preparation and Analytical Procedures
Each visit took place between 9 am and 5 pm in a nonfasting condition. Blood and tear fluid samples were collected following standardized protocols.16,18
For CGRP measurement, blood was collected in precooled 4 mL EDTA tubes (BD Vacutainer), which were previously prepared with 150 μL aprotinin (3–7 trypsin inhibitor unit (TIU)/mL) (Sigma Aldrich, Munich, Germany). The tubes were immediately centrifuged for 15 minutes at −6°C and 2,000 rpm. Plasma was then transferred in 1.5 mL polypropylene tubes (Eppendorf, Hamburg, Germany). We collected tear fluid from the lateral canthus of 1 eye with a 10-μL glass capillary (Brand, Wertheim, Germany). In participants with migraine, we selected the eye on the side on which migraine occurred most frequently. If there was no side preference and in participants without migraine, the right side was chosen by default. The capillary was removed after reaching the maximal volume of 10 μL or after 60 seconds at the latest. If the eye showed signs of irritation, such as redness or pruritus, the procedure was stopped immediately. A lack of tear production after 1 minute led to exclusion from the study. The volume of tear fluid collected was determined (range: 1.4–10.0 μL), and tear fluid was then transferred in a 1.5-mL tube containing 500 μL of tissue protein extractor solution (Pierce, Rockford, IL). Both plasma and tear fluid samples were stored at −80°C. We measured CGRP concentrations in plasma and tear fluid with a commercial sandwich ELISA kit (CUSABIO, Wuhan, China), following the manufacturer's instructions. The detection range of this kit is 1.56–100 pg/mL, and the minimal detectable dose was 0.39 pg/mL. However, the company does not disclose the specific recognition site of the ELISA antibodies. The kit has high intra-assay and interassay precision (coefficients of variation < 8% and <10%, respectively). Using this kit, mean CGRP concentrations in previous cohorts without migraine range from 4.2 to 6.6 pg/mL in plasma16,19-21 and between 0.7 and 0.8 ng/mL in the tear fluid.16,19
In addition, blood was collected in 5-mL serum tubes (BD Vacutainer) at room temperature and sent to our partner laboratory (Labor Berlin, Charité Vivantes GmbH) for the analysis of sex hormones. The following hormones were assessed via electrochemiluminescence immunoassay: estradiol, progesterone, testosterone, luteinizing hormone (LH), and follicle-stimulating hormone (FSH).
End Points
The primary end point of the study was the difference in CGRP concentrations in plasma (pg/mL) between M-RMC and C-RMC. Secondary end points were the differences in CGRP plasma concentrations between M-COC and C-COC and between M-PM and C-PM.
The differences in tear fluid CGRP concentrations (ng/mL) between the migraine and the control groups were considered exploratory endpoints. As further exploratory end points, we analyzed correlations between CGRP levels at both study visits in participants who were measured twice and assessed the differences in CGRP plasma and tear fluid concentrations among the 3 migraine and the 3 control groups. We also analyzed correlations between the estrogen and progesterone levels and the CGRP concentrations in tear fluid and plasma. In addition, the total cohort of participants with migraine was compared with the cohort of participants without migraine.
Statistical Analysis
Sample size calculation was performed using the software G*Power.22 Based on a previous study on interictal CGRP plasma levels in patients with migraine compared with controls without migraine,23 we assumed a large effect size of d = 0.8 for the primary end point. A sample size of 30 participants per group was therefore sufficient to detect an effect of similar magnitude with a statistical power of 0.80 at a significance level of α = 0.05 (2 tailed) using the Mann-Whitney U test. Similar statistical considerations apply for differences in tear fluid concentrations.16 We therefore aimed at 30 participants per group with complete data sets.
We summarized demographic, anamnestic, and laboratory data using descriptive statistics with median and interquartile ranges (IQRs) for numerical variables and frequencies and percentages for categorical variables. Given the non-normal data distribution, we compared outcomes between groups using the Mann-Whitney U test or the Kruskal-Wallis analysis of variance, as appropriate. Correlations were tested using Spearman rank correlations.
Statistical analysis was performed with SPSS Statistics 27 (IBM Corp., Armonk, NY). No adjustment for multiple comparisons was made for the exploratory outcome measures.
Standard Protocol Approvals, Registrations, and Patient Consents
The study protocol was approved by the Charité Ethical Committee (EA1/004/20). All participants provided written informed consent following study information.
Data Availability
Data not provided in the article because of space limitations may be shared (anonymized) at the request of any qualified investigator for purposes of replicating procedures and results.
Results
Between August 2020 and May 2022, n = 196 persons who self-identified as women participated in the study. The study protocol was completed by n = 180 female participants, n = 30 per group. Reasons for dropout were no sufficient lacrimation (n = 11), occurrence of migraine in the 12 hours after study visits with no possible rescheduling (n = 4), and lost to follow-up (n = 1).
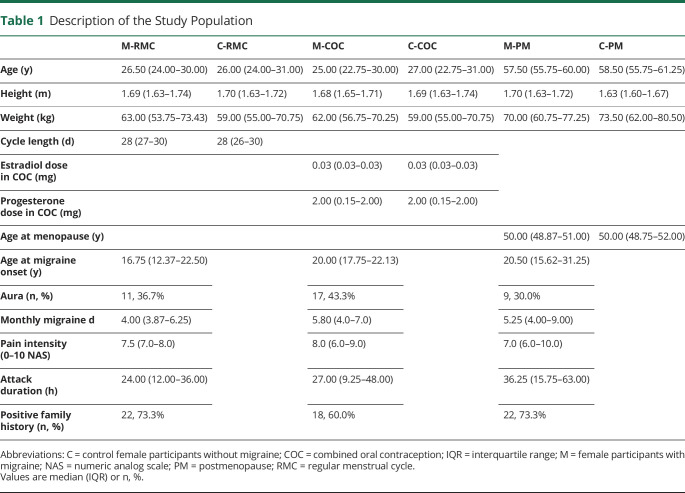
Demographic characteristics were similar between the migraine groups and the respective control groups. Table 1 shows the demographics across all groups and key migraine features in the 3 migraine groups. All female participants with migraine and an RMC reported migraine attacks within the perimenstrual period during most months.17
Table 1.
Description of the Study Population
Female Participants With a Regular Menstrual Cycle
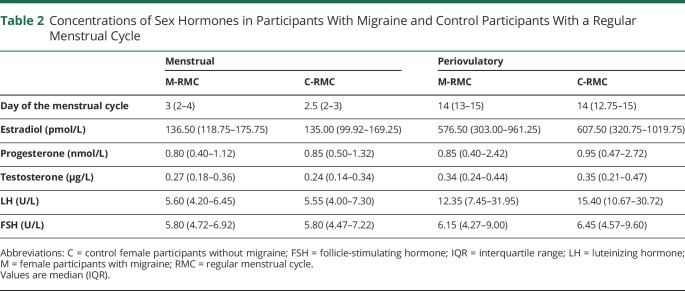
M-RMC and C-RMC presented physiologic hormonal levels at the 2 study visits with low estrogen concentrations during menstruation and high estrogen concentrations in the periovulatory period (Table 2). Progesterone levels were low at both time points because both visits occurred before the luteal progesterone increase (Table 2).
Table 2.
Concentrations of Sex Hormones in Participants With Migraine and Control Participants With a Regular Menstrual Cycle
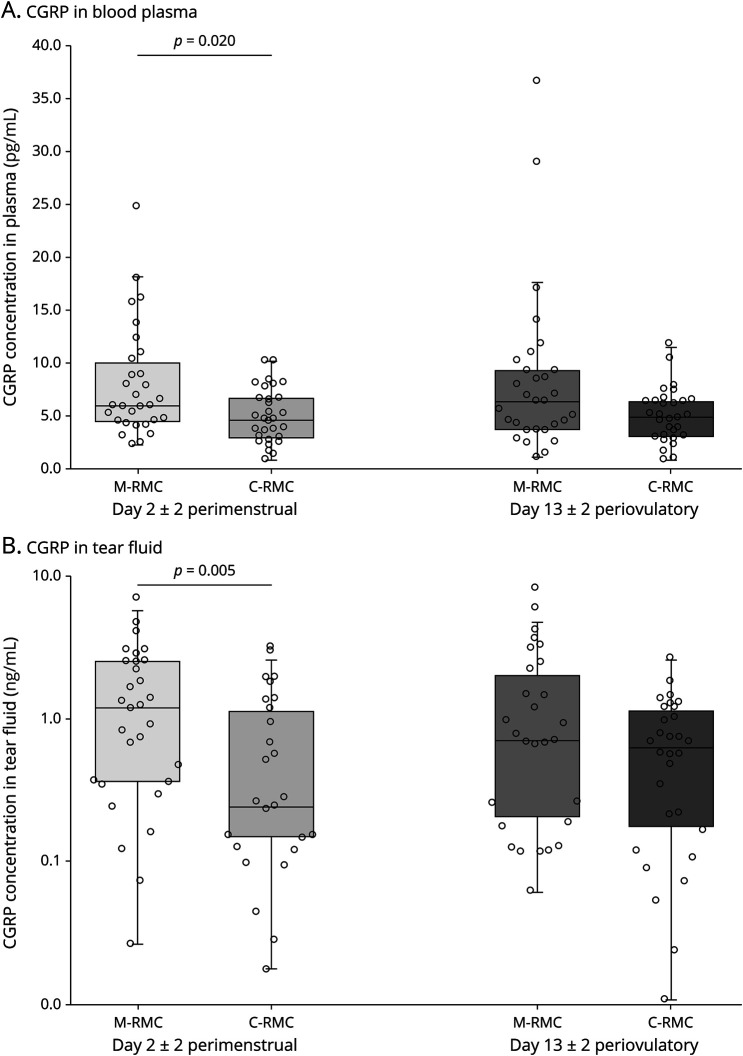
During menstruation, CGRP concentrations in both plasma and tear fluid were statistically significantly higher in interictal participants with migraine compared with female participants without migraine (plasma: 5.95 pg/mL [IQR 4.37–10.44 pg/mL] vs 4.61 pg/mL [IQR 2.83–6.92 pg/mL], p = 0.020; tear fluid: 1.20 ng/mL [IQR 0.36–2.52 ng/mL] vs 0.4 ng/mL [IQR 0.14–1.22 ng/mL], p = 0.005) (Figure 1).
Figure 1. CGRP Concentrations in Tear Fluid (A) and Plasma (B) in Participants With Migraine and Control Participants With a Regular Menstrual Cycle (RMC).
M = female participants with migraine; C = control female participants.
CGRP levels in the periovulatory period were numerically higher in female participants with migraine compared with participants without migraine but failed to reach statistical significance (plasma: 6.28 pg/mL [IQR 3.56–9.48 pg/mL] vs 4.87 pg/mL [IQR 2.95–6.41 pg/mL], p = 0.089; tear fluid: 0.70 ng/mL [IQR 0.18–2.29 ng/mL] vs 0.63 ng/mL [IQR 0.14–1.22 ng/mL], p = 0.225). There was a strong intraindividual correlation between the CGRP concentrations in the menstrual and the periovulatory visits, both in plasma (rho = 0.809, p < 0.001) and tear fluid (rho = 0.635, p < 0.001).
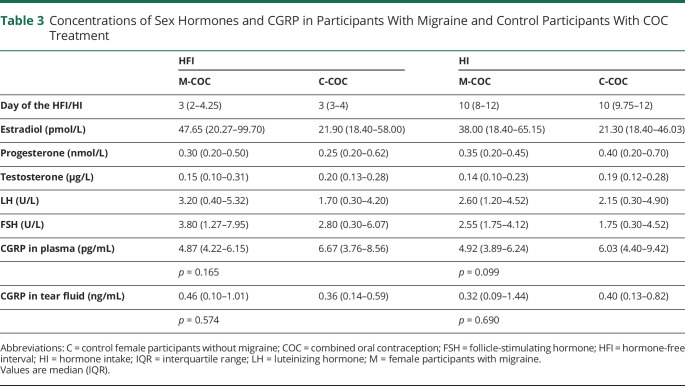
Female Participants With Combined Oral Contraception
Both M-COC and C-COC showed suppressed concentrations of naturally occurring sex hormones. CGRP concentrations in plasma and tear fluid were similar between participants with migraine and controls without migraine during the HFI and during HI (Table 3). There was a strong intraindividual correlation between the CGRP concentrations at both visits (plasma: rho = 0.797, p < 0.001; tear fluid: rho = 0.615, p < 0.001).
Table 3.
Concentrations of Sex Hormones and CGRP in Participants With Migraine and Control Participants With COC Treatment
Postmenopausal Female Participants
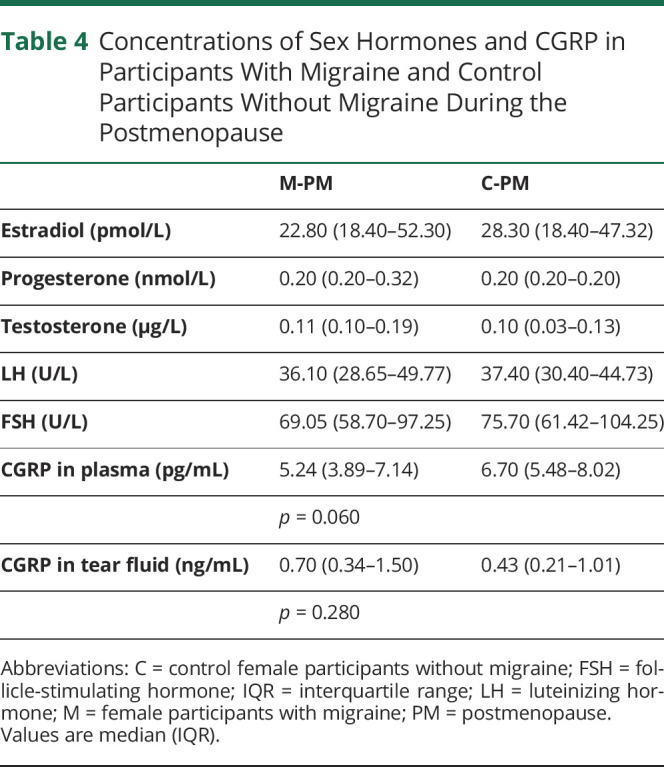
Both postmenopausal groups showed physiologic hormonal profiles with high concentrations of LH and FSH and low concentrations of estrogen, progesterone, and testosterone. There was no statistically significant difference in CGRP concentrations in plasma and tear fluid between M-PM and C-PM (Table 4).
Table 4.
Concentrations of Sex Hormones and CGRP in Participants With Migraine and Control Participants Without Migraine During the Postmenopause
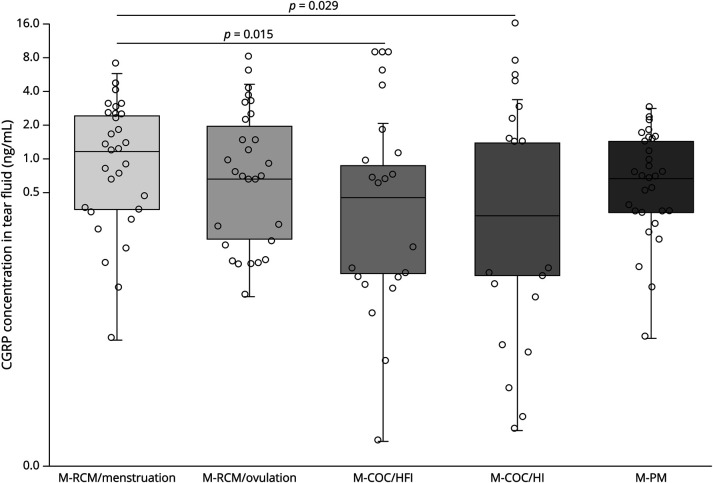
Comparison of CGRP Levels in Female Participants With Migraine in Different Hormonal States
Among all participants with migraine, CGRP plasma concentrations were similar among all groups and visits (p = 0.195 among all groups). In the tear fluid, female participants with an RMC had statistically significantly higher CGRP concentrations during menstruation compared with female participants under COC (p = 0.015 vs HFI and p = 0.029 vs HI) (Figure 2). There was no correlation between the absolute estrogen and progesterone concentrations and the CGRP concentrations in plasma and tear fluid (p > 0.17 for all analyses).
Figure 2. CGRP Tear Fluid Concentrations in Female Participants With Migraine in Different Hormonal States.
COC = combined oral contraception; HFI = hormone-free interval; HI = hormone intake; PM = postmenopause; RMC = regular menstrual cycle.
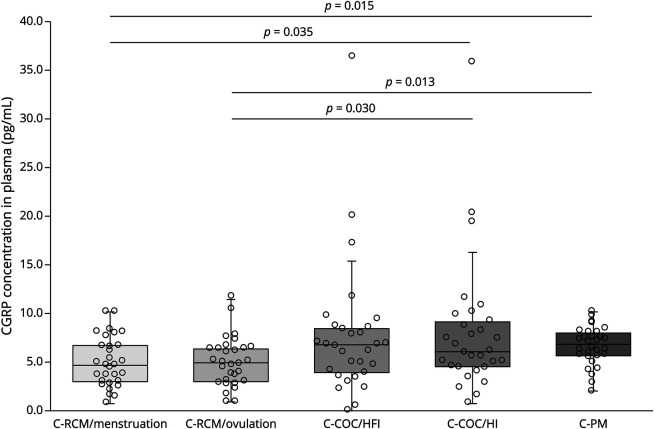
Comparison of CGRP Levels in Female Participants Without Migraine in Different Hormonal States
In plasma, CGRP concentrations of control female participants with an RMC were lower than those of female participants under COC treatment and postmenopausal female participants (menstruation vs HI: p = 0.035; ovulation vs HI: p = 0.030; menstruation vs postmenopause: p = 0.015; ovulation vs postmenopause: p = 0.013) (Figure 3). No statistically significant correlation between absolute sex hormone concentrations and CGRP concentrations could be detected (p > 0.17 for all analyses). CGRP levels in the tear fluid were similar across all groups and all visits of control female participants (p = 0.622 among all groups).
Figure 3. CGRP Plasma Concentrations in Female Participants Without Migraine in Different Hormonal States.
COC = combined oral contraception; HFI = hormone-free interval; HI = hormone intake; PM = postmenopause; RMC = regular menstrual cycle.
CGRP Plasma vs Tear Fluid Measurements
Across all participants (n = 180) and study visits (n = 300), CGRP concentrations were 5.48 pg/mL (3.98–7.82) in plasma and 0.51 ng/mL (0.16–1.22) in tear fluid. Tear fluid concentrations were 80.5× higher than in plasma (IQR 27.8–260.7).
Overall, participants with migraine had statistically significantly higher CGRP levels in tear fluid compared with participants without migraine (migraine groups: 0.67 ng/mL [IQR 0.17–1.59 ng/mL] vs control groups: 0.41 ng/mL [IQR 0.15–0.80 ng/mL], p = 0.013). Plasma concentrations were similar with 5.22 pg/mL (IQR 4.03–7.97 pg/mL) in the migraine groups vs 5.95 pg/mL (IQR 3.73–7.79 pg/mL) in the control groups (p = 0.965).
Discussion
CGRP levels in plasma and tear fluid in this large cohort of female participants varied depending on the presence of migraine and the hormonal status. Female participants with EM had higher interictal CGRP concentrations in plasma and the tear fluid during menstruation than female participants without migraine. This finding did not apply to female participants with COC and during the postmenopause. In female participants with migraine, the suppression of the hormonal fluctuations through COC treatment was associated with lower CGRP tear fluid levels than during physiologic menstruation.
Our findings suggest a link between sex hormones and CGRP in migraine pathophysiology in humans. The influence of sex hormones—in particular estrogen—on intracranial CGRP release has been studied mainly in vitro or animal research. Estrogen receptors are highly expressed in CGRP-positive neurons in the trigeminovascular system,24 and hormonal fluctuations can modulate their excitability.10,25 In animal models, deficiency of female sex hormones increases CGRP expression in various brain regions.26-28 Also in the trigeminal ganglion, the fall of endogenous estrogen levels in ovariectomized rats led to a significant increase in CGRP expression, which decreased following estrogen replacement treatment.29 These observations are in line with our results in female patients with migraine: the physiologic estrogen drop in the perimenstrual period was associated with higher CGRP concentrations than under hormonal contraceptive treatment.
A higher CGRP release during menstruation could help to explain the biological predisposition for more frequent, severe, and long-lasting migraine attacks in this period.30 In line with this hypothesis, menstrual migraine attacks were more frequent and severe than nonmenstrual attacks even in female persons treated with the CGRP receptor antibody erenumab.31 A recent review hypothesized that a decline in estrogen levels may lead to an increased CGRP signaling and generate a promigraine state with an increased susceptibility for migraine attacks.25 Of note, this seems to apply only for a decrease in naturally occurring estrogen concentrations coming from a previously higher level but not for stable low concentrations during the postmenopause. In addition, the absolute hormone concentrations do not seem to play a relevant role, but rather the changes in hormonal levels. Accordingly, all correlation analyses between estrogen or progesterone levels and CGRP concentrations did not reveal any statistically significant result.
A few older studies showed that sex hormones might affect CGRP concentrations also in individuals without migraine. A study from 1986 detected increased concentrations of immunoreactive CGRP in plasma during pregnancy, which decreased after delivery.11 In another older investigation, CGRP plasma levels were CGRP plasma levels were significantly higher in 11 female participants taking an oral contraception than in 12 female participants without hormonal treatment.12 The study did not provide data on the day of the menstrual cycle or the regimen of hormonal intake.12 In accordance with these results, in our study, oral contraception in female participants without migraine was associated with higher levels of CGRP in plasma but not in the tear fluid compared to fertile female participants without contraception. The intake of exogenous hormones seems to induce systemic changes in CGRP concentrations,10 whereas intracranial CGRP levels as indirectly measured in the tear fluid seem to be not affected. Indeed, high estrogen states like pregnancy have been demonstrated to increase CGRP concentrations in other anatomical regions such as the spinal cord.32 Estrogen substitution in rats led to a CGRP increase in the mesenteric arterioles, dorsal root ganglia,33,34 and in the gastric tract.35 Progesterone treatment induced an increased expression of CGRP receptors in the murine uterus and mesenteric arteries.36,37 The postmenopause is also associated with an increase in systemic CGRP levels,38 a finding which we could reproduce in our cohort of control female participants. The cardiovascular system has been proposed as the source of the elevated CGRP concentrations, as postmenopausal female persons with vasomotor symptoms appear particularly affected.39,40 Taken together, hormone-dependent CGRP changes in plasma of female persons without migraine seem to originate from sources other than the trigeminovascular system.
CGRP concentrations in plasma are influenced by a multitude of factors and allow limited conclusions about the release from the trigeminal nerve system.15 It is estimated that only one-fifth of CGRP in peripheral blood derives from trigeminal sources.16 Although the crucial role of CGRP in migraine pathophysiology is indisputable, the feasibility of plasma CGRP as a biomarker of migraine remains a matter of debate.15 Previous research reported controversial results regarding interictal plasma CGRP levels in patients with EM: although some studies detected higher CGRP levels in cubital vein blood outside of acute migraine attacks, others observed no difference to controls without migraine.23,41-43 Our results provide a differentiated view depending on the hormonal status of the patients. Female participants with EM during menstruation had higher interictal plasma CGRP concentrations than female participants without EM, whereas this was not the case in the other hormonal conditions examined.
Biomaterials closer to the trigeminal CGRP source such as tear fluid may represent a more direct and suitable approach.16 A recent study reported, in n = 30 interictal mix-sexed patients with EM, higher CGRP concentrations than in n = 48 controls without EM.16 In the current analysis, we could confirm and expand these findings to a significantly larger cohort. Similar to this previous study, CGRP levels in the tear fluid were much higher than in plasma possibly due to lower proteolytic activity in this liquid than in plasma. In fact, in individuals without ophthalmologic conditions, the levels of peptidases are generally low in the tear fluid.44-46 On the contrary, CGRP in plasma is quickly sheared into shorter fragments by endopeptidases,47 which may in part explain the lower CGRP concentrations detected with a commercial ELISA. More complex methods such as high-performance liquid chromatography are able to detect and differentiate between different peptide fragments.47
CGRP in the tear fluid originates mainly from trigeminal nerve fibers in the cornea and conjunctiva, whereas ocular autonomic nerve fibers and the lacrimal and meibomian glands express only little or no CGRP.48,49 Averaged over the whole cohort, the median CGRP concentrations in the tear fluid of interictal patients with migraine were higher than in controls without migraine. This corroborates the hypothesis of an increased activation of the trigeminovascular system even outside the acute attacks. However, in the analysis by subgroups, statistical significance was confirmed only in menstruating persons. Future studies should therefore take the hormonal status of the participants into account when examining CGRP in migraine. Despite these promising findings, CGRP determination in the tear fluid lacks validation and should be considered an exploratory procedure. For further use, a thorough validation study needs to be performed to compare the performance characteristics of CGPR levels in the tear fluid with the current standard measurement in plasma.
This is a comprehensive analysis of sex hormones and CGRP concentrations in female persons with migraine. The 3 groups of female participants with migraine were similar regarding migraine frequency and intensity. The selection of age-matched female participants without migraine and without other significant diseases or regular medication represents a key strength of this investigation. The measurement of sex hormone concentrations at each visit ensured that participants were in the predefined hormonal phase. Without a continuous hormonal measurement, however, we cannot determine whether the periovulatory visits took place exactly on the day of ovulation or rather in the few days before or after. Of note, we excluded female persons with a pure menstrual migraine, who might possibly have an even stronger influence of hormonal fluctuations on migraine-inducing mechanisms. Moreover, we included only cisgender women. Therefore, the findings do not generalize to all women (e.g., transgender women). One further limitation is the definition of the interictal state, that is, at least 12 hours free of migraine and acute medication before and after each visit. This is shorter than in other similar investigations.16 We rationalized that the shortening of this period reduces organizational visit changes and thereby dropouts. Twelve hours is more than 2 elimination half-lives of most triptans and NSAIDs, and we did not expect any relevant residual efficacy after this time.50 CGRP measurement requires strict preanalytical sample handling, and CGRP concentrations may vary between studies depending on the exact methodology. In this study, we followed the protocol by Kamm et al. (2019) with the most sensitive commercial ELISA kit that is available. Indeed, we found similar concentrations of CGRP in both plasma and tear fluid as described in this previous study and other studies with the same commercial kit.16,19-21 The detection of a strong correlation of CGRP levels between study visits in participants that were assessed twice proves a high interindividual consistency. Importantly, multiple physiologic and pathologic processes can influence both CGRP and sex hormone concentrations. Despite careful selection of subjects and standardized visits, we could not control for all possible confounding factors. This study is intended as a pilot study. It provides evidence of an association between CGRP and different sex hormone profiles in humans and sets the context for further studies with larger sample sizes and adequate power to correct for multiple testing and confounders.
In conclusion, our data suggest hormone-dependent changes in CGRP concentrations in female patients with EM. The elevated CGRP release from the trigeminovascular system following hormonal fluctuations could help to explain a higher susceptibility for migraine in female people who menstruate. The lower CGRP tear fluid concentrations under hormonal contraception in patients with migraine could be associated with an altered migraine susceptibility under hormonal therapy and should be further investigated in a longitudinal design.
Editors' Note
Neurology recognizes that sex and gender are not interchangeable. Neurology editors aim to ensure that papers accurately describe and report which of these variables was evaluated in a study. In this case, the authors included only female participants, and this is the terminology used throughout the paper. We were unable to find an equivalent term to use in the title, as style guidelines suggest against using “females” as a noun. Since all the participants also identified as women, we made an editorial decision to use women in the title. Neurology strives to affirm persons of all genders and recognizes that the findings of this article may not pertain to all persons who identify as women.
Rebecca Burch, MD; Roy H. Hamilton, MD, MS; Holly E. Hinson, MD, MCR
Glossary
- C-COC
without EM–treatment with a COC
- CGRP
calcitonin gene-related peptide
- COC
combined oral contraceptive
- C-PM
without EM–postmenopause
- C-RMC
without EM–regular menstrual cycle
- EM
episodic migraine
- FSH
follicle-stimulating hormone
- HFI
hormone-free interval
- HI
hormone intake
- IQR
interquartile range
- LH
luteinizing hormone
- M-COC
with EM–treatment with a COC
- M-PM
with EM–postmenopause
- M-RMC
with EM–regular menstrual cycle
Appendix. Authors
Footnotes
Patient Page e1849
Study Funding
The authors report no targeted funding.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Burch RC, Buse DC, Lipton RB. Migraine: epidemiology, burden, and comorbidity. Neurol Clin. 2019;37(4):631-649. 10.1016/j.ncl.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 2.Martin VT, Lipton RB. Epidemiology and biology of menstrual migraine. Headache. 2008;48(48 suppl):S124-S130. doi. 10.1111/j.1526-4610.2008.01310.x. [DOI] [PubMed] [Google Scholar]
- 3.Somerville BW. The role of estradiol withdrawal in the etiology of menstrual migraine. Neurology. 1972;22(4):355-365. doi. 10.1212/wnl.22.4.355. [DOI] [PubMed] [Google Scholar]
- 4.Delaruelle Z, Ivanova TA, Khan S, et al. Male and female sex hormones in primary headaches. J Headache Pain. 2018;19(1):117. doi. 10.1186/s10194-018-0922-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.MacGregor EA. Migraine, menopause and hormone replacement therapy. Post Reprod Health. 2018;24(1):11-18. doi. 10.1177/2053369117731172. [DOI] [PubMed] [Google Scholar]
- 6.MacGregor EA. Contraception and headache. Headache. 2013;53(2):247-276. doi. 10.1111/head.12035. [DOI] [PubMed] [Google Scholar]
- 7.United Nations–Department of Economic and Social Affairs. Contraceptive use by method; 2019. un.org/development/desa/pd/sites/www.un.org.development.desa.pd/files/files/documents/2020/Jan/un_2019_contraceptiveusebymethod_databooklet.pdf.Accessed August 9, 2022.
- 8.Edvinsson L, Haanes KA, Warfvinge K, Krause DN. CGRP as the target of new migraine therapies–successful translation from bench to clinic. Nat Rev Neurol. 2018;14(6):338-350. doi. 10.1038/s41582-018-0003-1. [DOI] [PubMed] [Google Scholar]
- 9.Goadsby PJ, Edvinsson L, Ekman R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann Neurol. 1990;28(2):183-187. doi. 10.1002/ana.410280213. [DOI] [PubMed] [Google Scholar]
- 10.Labastida-Ramírez A, Rubio-Beltrán E, Villalón CM, MaassenVanDenBrink A. Gender aspects of CGRP in migraine. Cephalalgia. 2019;39(3):435-444. doi. 10.1177/0333102417739584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stevenson JC, Macdonald DW, Warren RC, Booker MW, Whitehead MI. Increased concentration of circulating calcitonin gene related peptide during normal human pregnancy. Br Med J (Clin Res Ed). 1986;293(6558):1329-1330. doi. 10.1136/bmj.293.6558.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Valdemarsson S, Edvinsson L, Hedner P, Ekman R. Hormonal influence on calcitonin gene-related peptide in man: effects of sex difference and contraceptive pills. Scand J Clin Lab Invest. 1990;50(4):385-388. doi. 10.3109/00365519009091595. [DOI] [PubMed] [Google Scholar]
- 13.Ibrahimi K, Vermeersch S, Danser AHJ, et al. Development of an experimental model to study trigeminal nerve-mediated vasodilation on the human forehead. Cephalalgia. 2014;34(7):514-522. doi. 10.1177/0333102413517773. [DOI] [PubMed] [Google Scholar]
- 14.Ibrahimi K, Vermeersch S, Frederiks P, et al. The influence of migraine and female hormones on capsaicin-induced dermal blood flow. Cephalalgia. 2016;37(12):1164-1172. doi. 10.1177/0333102416668659. [DOI] [PubMed] [Google Scholar]
- 15.Lee MJ, Lee SY, Cho S, Kang ES, Chung CS. Feasibility of serum CGRP measurement as a biomarker of chronic migraine: a critical reappraisal. J Headache Pain. 2018;19(1):53. doi. 10.1186/s10194-018-0883-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kamm K, Straube A, Ruscheweyh R. Calcitonin gene-related peptide levels in tear fluid are elevated in migraine patients compared to healthy controls. Cephalalgia. 2019;39(12):1535-1543. doi. 10.1177/0333102419856640. [DOI] [PubMed] [Google Scholar]
- 17.Headache classification committee of the international headache society (IHS) the international classification of headache disorders, 3rd edition. Cephalalgia. 2018;38(1):1-211. [DOI] [PubMed] [Google Scholar]
- 18.Raffaelli B, Overeem LH, Mecklenburg J, et al. Plasma calcitonin gene-related peptide (CGRP) in migraine and endometriosis during the menstrual cycle. Ann Clin Transl Neurol. 2021;8(6):1251-1259. doi. 10.1002/acn3.51360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamm K, Straube A, Ruscheweyh R. Baseline tear fluid CGRP is elevated in active cluster headache patients as long as they have not taken attack abortive medication. Cephalalgia. 2021;41(1):69-77. doi. 10.1177/0333102420949858. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Z, Gong F, Lu GX. Plasma level of calcitonin gene-related peptide in patients with polycystic ovary syndrome and its relationship to hormonal and metabolic parameters. Peptides. 2012;34(2):343-348. doi. 10.1016/j.peptides.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 21.Gárate G, Pascual M, Olmos JM, et al. Increase in serum calcitonin gene-related peptide β (CGRPβ) levels in COVID-19 patients with diarrhea: an underlying mechanism? Dig Dis Sci. 2022;67(12):5712-5713. doi. 10.1007/s10620-022-07473-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175-191. doi. 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 23.Cernuda-Morollón E, Larrosa D, Ramón C, Vega J, Martinez-Camblor P, Pascual J. Interictal increase of CGRP levels in peripheral blood as a biomarker for chronic migraine. Neurology. 2013;81(14):1191-1196. doi. 10.1212/wnl.0b013e3182a6cb72. [DOI] [PubMed] [Google Scholar]
- 24.Warfvinge K, Krause DN, Maddahi A, Edvinsson JCA, Edvinsson L, Haanes KA. Estrogen receptors α, β and GPER in the CNS and trigeminal system–molecular and functional aspects. J Headache Pain. 2020;21(1):131. doi. 10.1186/s10194-020-01197-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krause DN, Warfvinge K, Haanes KA, Edvinsson L. Hormonal influences in migraine–interactions of oestrogen, oxytocin and CGRP. Nat Rev Neurol. 2021;17(10):621-633. doi. 10.1038/s41582-021-00544-2. [DOI] [PubMed] [Google Scholar]
- 26.Wang D, Zhao J, Wang J, Li J, Yu S, Guo X. Deficiency of female sex hormones augments PGE2 and CGRP levels within midbrain periaqueductal gray. J Neurol Sci. 2014;346(1-2):107-111. doi. 10.1016/j.jns.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 27.Herbison AE, Spratt DP. Sexually dimorphic expression of calcitonin gene-related peptide (CGRP) mRNA in rat medial preoptic nucleus. Brain Res Mol Brain Res. 1995;34(1):143-148. doi. 10.1016/0169-328x(95)00144-h. [DOI] [PubMed] [Google Scholar]
- 28.Yang Y, Ozawa H, Lu H, et al. Immunocytochemical analysis of sex differences in calcitonin gene-related peptide in the rat dorsal root ganglion, with special reference to estrogen and its receptor. Brain Res. 1998;791(1-2):35-42. doi. 10.1016/s0006-8993(98)00021-3. [DOI] [PubMed] [Google Scholar]
- 29.Aggarwal M, Puri V, Puri S. Effects of estrogen on the serotonergic system and calcitonin gene-related peptide in trigeminal ganglia of rats. Ann Neurosci. 2012;19(4):151-157. doi. 10.5214/ans.0972.7531.190403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.MacGregor EA. Menstrual and perimenopausal migraine: a narrative review. Maturitas. 2020;142:24-30. doi. 10.1016/j.maturitas.2020.07.005. [DOI] [PubMed] [Google Scholar]
- 31.Ornello R, Frattale I, Caponnetto V, De Matteis E, Pistoia F, Sacco S. Menstrual headache in women with chronic migraine treated with erenumab: an observational case series. Brain Sci. 2021;11(3):370. doi. 10.3390/brainsci11030370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mowa CN, Usip S, Collins J, Storey-Workley M, Hargreaves K, Papka R. The effects of pregnancy and estrogen on the expression of calcitonin gene-related peptide (CGRP) in the uterine cervix, dorsal root ganglia and spinal cord. Peptides. 2003;24(8):1163-1174. doi. 10.1016/j.peptides.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 33.Blacklock AD, Cauveren JA, Smith PG. Estrogen selectively increases sensory nociceptor innervation of arterioles in the female rat. Brain Res. 2004;1018(1):55-65. doi. 10.1016/j.brainres.2004.05.075. [DOI] [PubMed] [Google Scholar]
- 34.Gangula PR, Chauhan M, Reed L, Yallampalli C. Age-related changes in dorsal root ganglia, circulating and vascular calcitonin gene-related peptide (CGRP) concentrations in female rats: effect of female sex steroid hormones. Neurosci Lett. 2009;454(2):118-123. doi. 10.1016/j.neulet.2009.02.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang X, Liu R, Dong Y. Regulative effects of ovarian steroids on rat gastric motility and sensitivity. Sheng Li Xue Bao. 2006;58(3):275-280. [PubMed] [Google Scholar]
- 36.Yallampalli C, Gangula PR, Kondapaka S, Fang L, Wimalawansa S. Regulation of calcitonin gene-related peptide receptors in the rat uterus during pregnancy and labor and by progesterone. Biol Reprod. 1999;61(4):1023-1030. doi. 10.1095/biolreprod61.4.1023. [DOI] [PubMed] [Google Scholar]
- 37.Yallampalli C, Kondapaka SB, Lanlua P, Wimalawansa S, Gangula P. Female sex steroid hormones and pregnancy regulate receptors for calcitonin gene-related peptide in rat mesenteric arteries, but not in aorta. Biol Reprod. 2004;70(4):1055-1062. doi. 10.1095/biolreprod.103.022467. [DOI] [PubMed] [Google Scholar]
- 38.Gupta P, Harte A, Sturdee DW, et al. Effects of menopausal status on circulating calcitonin gene-related peptide and adipokines: implications for insulin resistance and cardiovascular risks. Climacteric. 2008;11(5):364-372. doi. 10.1080/13697130802378493. [DOI] [PubMed] [Google Scholar]
- 39.Wyon Y, Frisk J, Lundeberg T, Theodorsson E, Hammar M. Postmenopausal women with vasomotor symptoms have increased urinary excretion of calcitonin gene-related peptide. Maturitas. 1998;30(3):289-294. doi. 10.1016/s0378-5122(98)00047-4. [DOI] [PubMed] [Google Scholar]
- 40.Wyon YAM, Spetz ACE, Theodorsson GE, Hammar ML. Concentrations of calcitonin gene-related peptide and neuropeptide Y in plasma increase during flushes in postmenopausal women. Menopause. 2000;7(1):25-30. doi. 10.1097/00042192-200007010-00005. [DOI] [PubMed] [Google Scholar]
- 41.Ashina M, Bendtsen L, Jensen R, Schifter S, Olesen J. Evidence for increased plasma levels of calcitonin gene-related peptide in migraine outside of attacks. Pain. 2000;86(1):133-138. doi. 10.1016/s0304-3959(00)00232-3. [DOI] [PubMed] [Google Scholar]
- 42.Gupta R, Ahmed T, Banerjee B, Bhatia M. Plasma calcitonin gene-related peptide concentration is comparable to control group among migraineurs and tension type headache subjects during inter-ictal period. J Headache Pain. 2009;10(3):161-166. doi. 10.1007/s10194-009-0110-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gallai V, Sarchielli P, Floridi A, et al. Vasoactive peptide levels in the plasma of young migraine patients with and without aura assessed both interictally and ictally. Cephalalgia. 1995;15(5):384-390. doi. 10.1046/j.1468-29821995.1505384.x. [DOI] [PubMed] [Google Scholar]
- 44.Määttä M, Kari O, Tervahartiala T, et al. Tear fluid levels of MMP-8 are elevated in ocular rosacea–treatment effect of oral doxycycline. Graefes Arch Clin Exp Ophthalmol. 2006;244(8):957-962. doi. 10.1007/s00417-005-0212-3. [DOI] [PubMed] [Google Scholar]
- 45.Virtanen T, Konttinen YT, Honkanen N, Harkonen M, Tervo T. Tear fluid plasmin activity of dry eye patients with Sjögren's syndrome. Acta Ophthalmol Scand. 2009;75(2):137-141. doi. 10.1111/j.1600-0420.1997.tb00109.x. [DOI] [PubMed] [Google Scholar]
- 46.Cejková J, Zvárová Z, Cejka C. Dipeptidyl peptidase IV (DPPIV) activity in the tear fluid as an indicator of the severity of corneal injury: a histochemical and biochemical study. Histol Histopathol. 2004;19(3):669-676. doi. 10.14670/HH-19.669. [DOI] [PubMed] [Google Scholar]
- 47.Edvinsson L, Ekman R, Goadsby PJ. Measurement of vasoactive neuropeptides in biological materials: problems and pitfalls from 30 years of experience and novel future approaches. Cephalalgia. 2010;30(6):761-766. doi. 10.1177/0333102409351807. [DOI] [PubMed] [Google Scholar]
- 48.Elsås T, Edvinsson L, Sundler F, Uddman R. Neuronal pathways to the rat conjunctiva revealed by retrograde tracing and immunocytochemistry. Exp Eye Res. 1994;58(1):117-126. doi. 10.1006/exer.1994.1201. [DOI] [PubMed] [Google Scholar]
- 49.Jones MA, Marfurt CF. Peptidergic innervation of the rat cornea. Exp Eye Res. 1998;66(4):421-435. doi. 10.1006/exer.1997.0446. [DOI] [PubMed] [Google Scholar]
- 50.Jhee SS, Shiovitz T, Crawford AW, Cutler NR. Pharmacokinetics and pharmacodynamics of the triptan antimigraine agents: a comparative review. Clin Pharmacokinet. 2001;40(3):189-205. doi. 10.2165/00003088-200140030-00004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data not provided in the article because of space limitations may be shared (anonymized) at the request of any qualified investigator for purposes of replicating procedures and results.