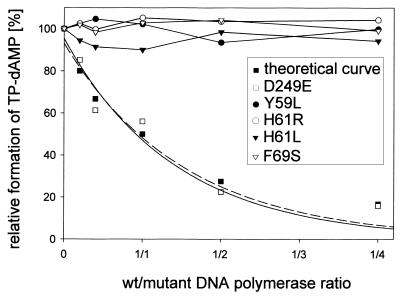
Figure 5.
Mutant Φ29 DNA polymerases Y59L, H61R/L and F69S do not interfere with wild-type polymerase for TP interaction. Reactions were carried out as described for the template-independent initiation assay using a limiting amount of TP and different proportions (5, 10, 25, 50 or 100 ng) of mutant DNA polymerases Y59L, H61R, H61L and F69S, respectively. The amount of TP–dAMP formed under competition conditions is followed relative to the amount formed in the absence of competitor. An inactive polymerase with unmodified TP interaction ability should inhibit formation of the TP–dAMP complex by competition with the wild-type enzyme. None of the indicated mutants showed this behavior. As a control for 100% inhibition, the previously analyzed mutant D249E was used (32), whose inhibition profile resembles the theoretical one. The lines for theoretical and mutant D249E values are least squares fits to a simple exponential decay.