Figure 2. Monoaxial and Coaxial Ace-DEX MP adjuvants protect equally against lethal influenza challenge in mice:
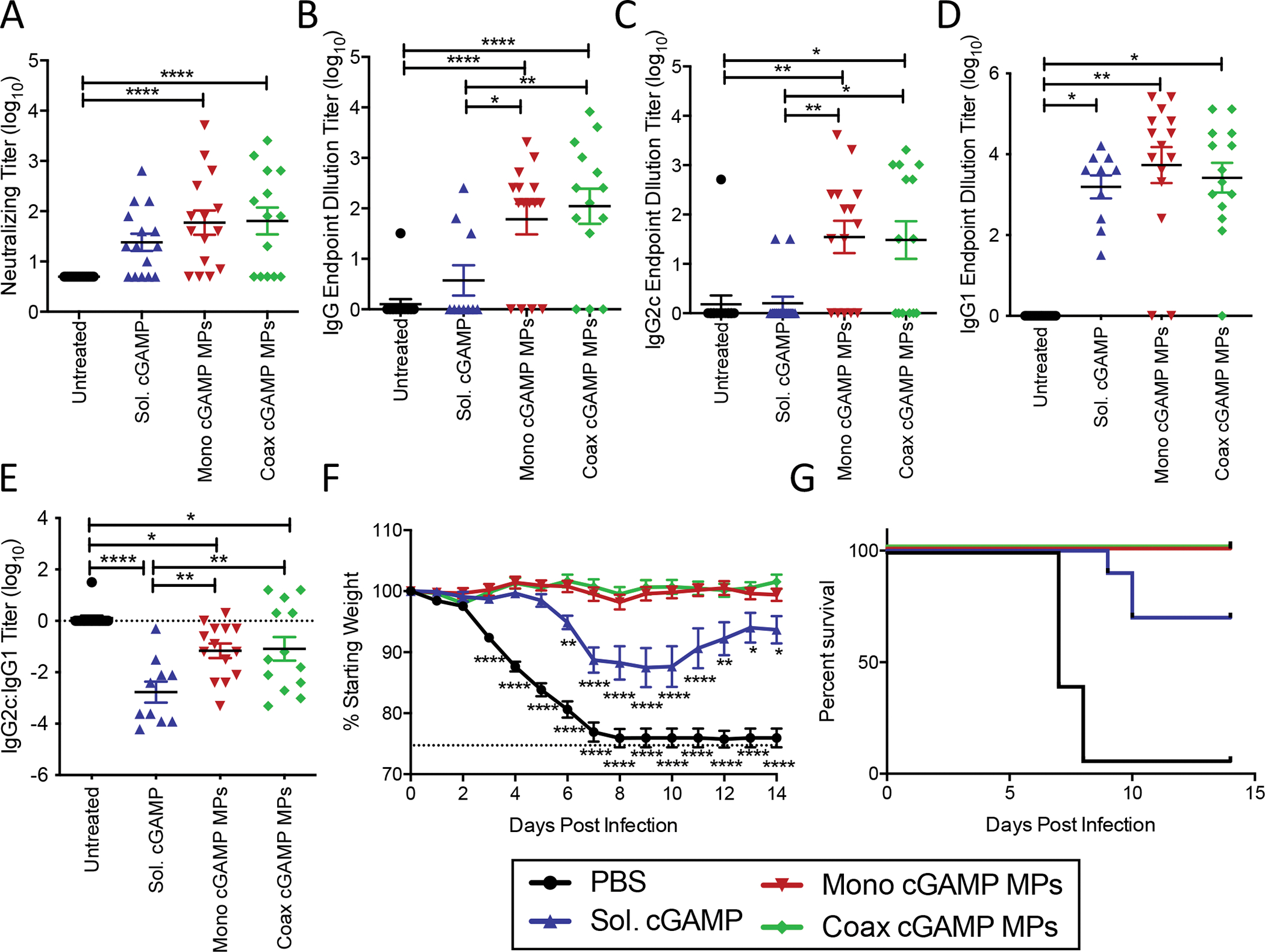
Eight-week-old female C57BL/6 mice were immunized i.m. with PBS, or recombinant HA from influenza strain A/Puerto Rico/8/34 (1 μg) adjuvanted with soluble cGAMP (0.2 μg), or Ace-DEX cGAMP MPs (0.2 μg cGAMP in 20 μg MPs) formulated through coaxial (coax) or monoaxial (mono) electrospray. Animals received a boost with the same formulation 21 days post-prime. (A) Serum was collected 28 days post-prime and virus neutralizing titers against A/Puerto Rico/8/34 were assessed. (C-D) The serum antibody endpoint titers at day 28 of HA specific (B) total IgG, (C) anti-viral Th1-skewed IgG2c, and (D) Th2-skewed IgG1 antibody isotypes were also assessed. (E) The ratio of IgG2c:IgG1 titers. (F-G) Following challenge with 5,000 ffu of influenza strain A/Puerto Rico/8/34, (F) weight loss, (G) and survival were monitored for 14 days. (n=10=15 ± SEM, *p < 0.05, **p < 0.01, ****p < 0.0001).
