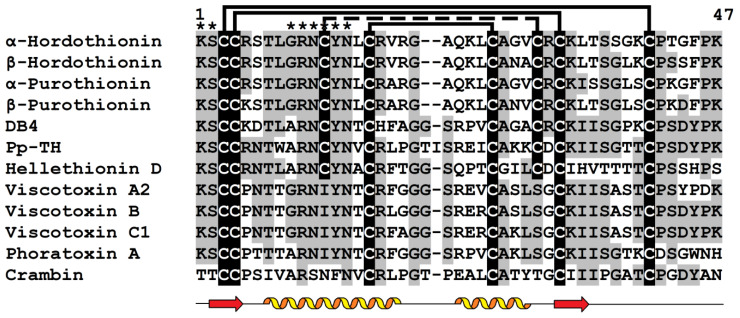
Figure 1.
Multiple sequence alignment of selected thionins: α-hordothionin (GenBank AAA32966.1) and β-hordothionin (GenBank 1206255A) from Hordeum vulgare; α-purothionin (GenBank CAA65313.1) and β-purothionin (GenBank CAA65312.1) from Triticum aestivum, leaf-specific thionin DB4 from H. vulgare (UniProt P08772.2); Pp-TH from Pyrularia pubera (UniProt P07504.1); hellethionin D from Helleborus purpurascens (UniProt P60057.1); viscotoxin А2 (UniProt P32880.1), В (GenBank P08943.2), and С1 (UniProt P83554.1) from Viscum album; phoratoxin А from California mistletoe (UniProt P01539.1); crambin from Crambe abyssinica (UniProt P01542.2). Cysteine residues are highlighted in white on the black background. Conserved amino acid residues are highlighted in black on the grey background. Lines above the sequences denote disulfide bonds. Asterisks indicate conserved amino acid residues supposed to be involved in interactions with phospholipids. Secondary structure elements (α-heliсеs and β-strands) for β-purothionin (PDB 1BHP) are shown under the alignment as helices and arrows, respectively.