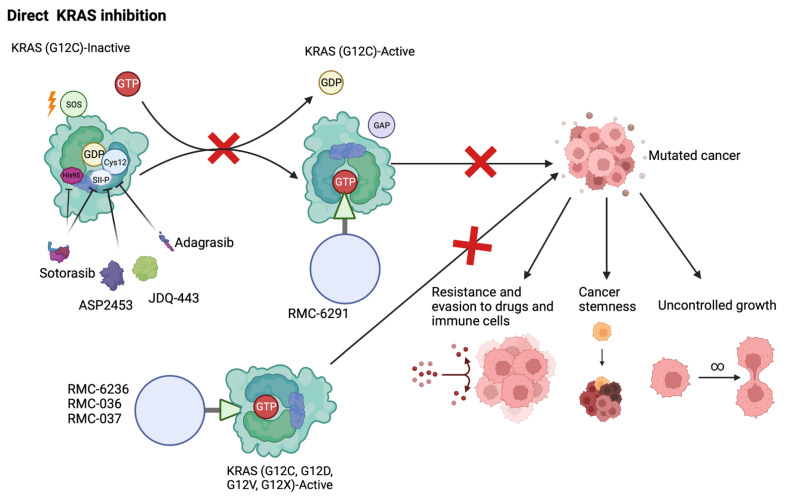
Figure 2.
Direct inhibition of KRAS G12C mutation. The listed drugs Sotorasib, ASP2453, JDQ443, and Adagrasib work through a similar mechanism, binding to the mutated Cys12 residue of KRAS mutants. By making contacts with the Switch II pocket (SII-P) region, the inhibitors make a stable connection with KRAS and prevent structural change into its active conformation. Sotorasib uniquely binds to a His95 residue proximal to the SII-P to increase contact with the protein. In doing so, the inhibitors subdue tumorigenic phenotypes including cancer stemness, uncontrolled proliferation, and the ability to evade immune and drug attack. The series of inhibitors from Revolution Medicines, RMC-6291, RMC-6236, RMC-036, and RMC-037, bind instead to the GTP-bound active form of KRAS. Creating a tri-compound structure with Cyclophilin A, the inhibitors can bind G12C and other mutant KRAS proteins.