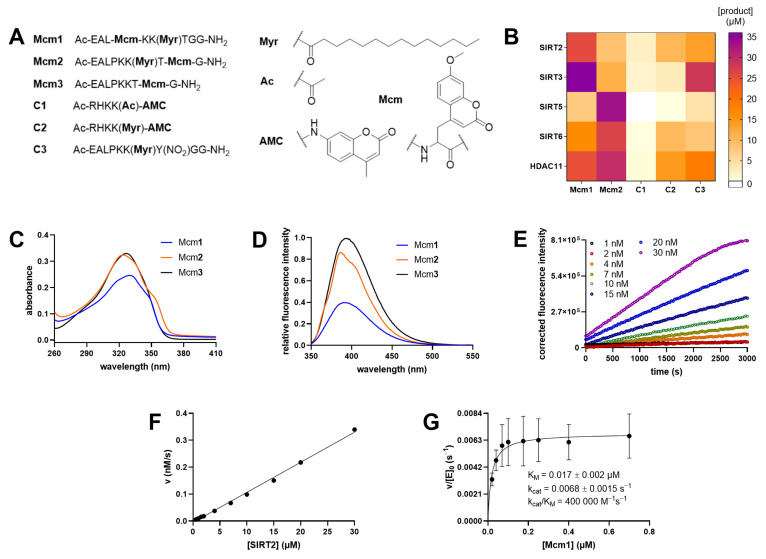
Figure 2.
Direct and continuous activity assay for demyristoylation using the environmentally sensitive fluorophore Mcm (7-Methoxycoumarin-4-yl-alanyl). (A) Structures of compounds Mcm1 to Mcm3 and control peptides C1 to C3. Y(NO2) corresponded to meta-nitrotyrosine. (B) Peptide substrates at an initial concentration of 50 µM were treated with either 50 nM HDAC11, or with SIRT2 (0.1 µM), SIRT3 (0.1 µM), SIRT5 (0.5 µM), or SIRT6 (0.5 µM) in the presence of 500 µM NAD+ at 37 °C for 60 min. Product formation was monitored via HPLC at 320 nm or 360 nm for C3. Values were obtained from three independent replicates. (C) Absorbance spectra for Mcm1 to Mcm3 at 30 µM concentration. (D) Normalized fluorescence spectra of Mcm1 to Mcm3 at 3 µM concentration. (E) Progress curves of Mcm1 deacylation (1 µM) by SIRT2 (1nM-30nM) at 25 °C (λEx = 330 ± 75 nm and λEm = 405 ± 8 nm) monitored in a 96-well plate format. (F) The reaction rate shows a linear correlation with the SIRT2 concentration. (G) Michaelis-Menten kinetic analysis of Mcm1 deacylation by SIRT2 obtained from three independent replicates.