Figure 1. Enrollment and Follow-up of the Study Participants.
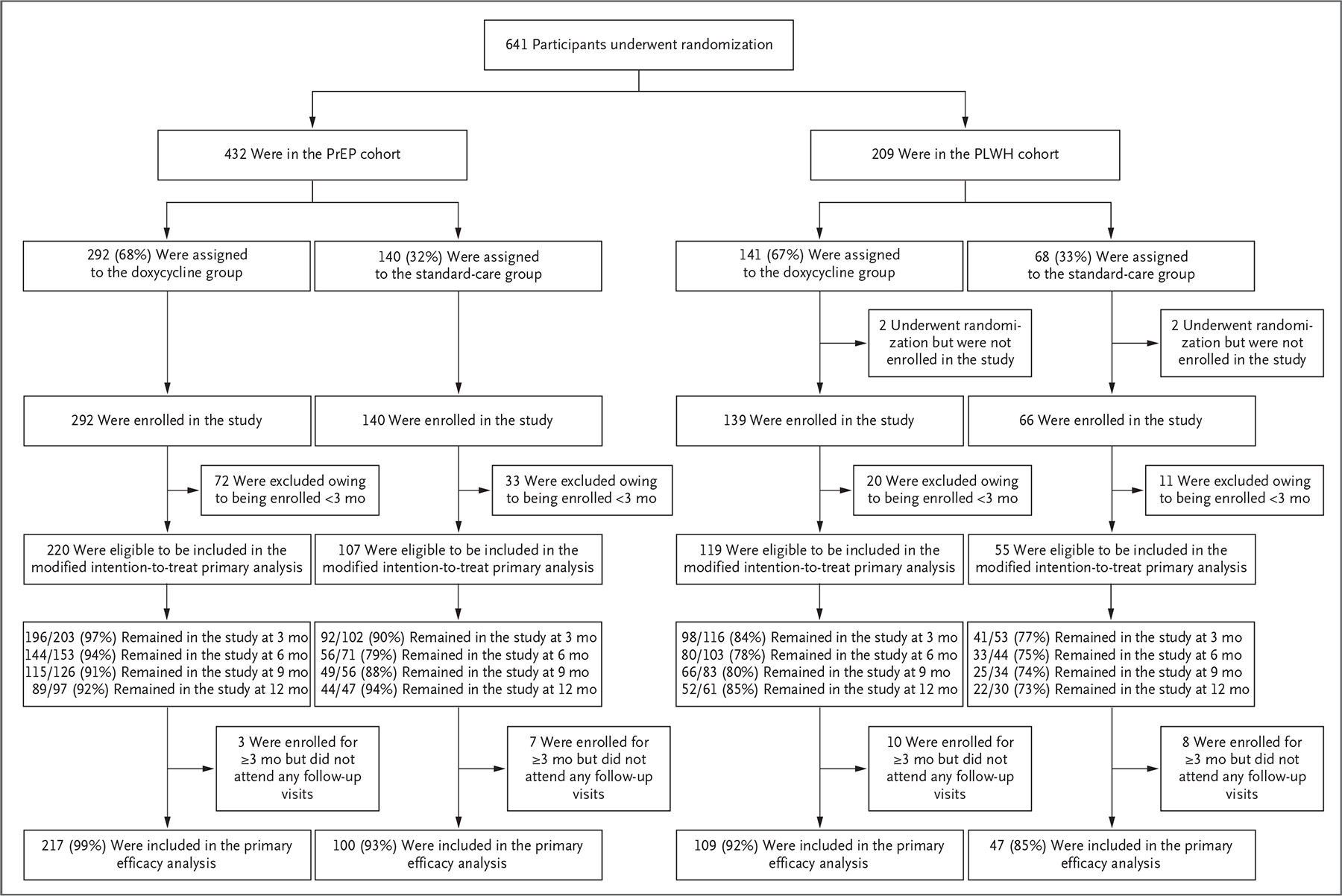
There was no separate screening visit; thus, reasons for screening failure were not collected. The study had two cohorts: those who were taking preexposure prophylaxis (PrEP) against human immunodeficiency virus (HIV) infection (PrEP cohort) and persons living with HIV infection (PLWH cohort). Within each cohort, participants in the doxycycline group were assigned to take doxycycline within 72 hours after condomless sex (doxycycline postexposure prophylaxis [doxy-PEP]), and participants in the standard-care group were assigned to receive standard care without doxycycline. Of the 4 participants in the PLWH cohort who underwent randomization but were not enrolled in the study, 3 immediately withdrew consent after receiving their randomization assignment and 1 was withdrawn at the investigator’s discretion. A total of 136 participants were enrolled but had not yet reached the month 3 visit at the time of this analysis. Visit attendance indicates the percentage who had completed visits for which they were eligible at the time that the data and safety monitoring board met. A total of 28 participants remained in follow-up but had not completed a follow-up visit. A total of 473 of 501 participants (94%) in the modified intention-to-treat population contributed follow-up visit data for the primary efficacy analysis. A total of 18 participants discontinued the study early: 5 in the doxycycline groups (1 moved, 1 had a new job, 1 was in a monogamous relationship, and 2 gave no reason) and 13 in the standard-care groups (2 moved, 1 had a new job, 6 wanted doxy-PEP, 1 had a concern about coronavirus disease 2019, and 3 gave no reason).
