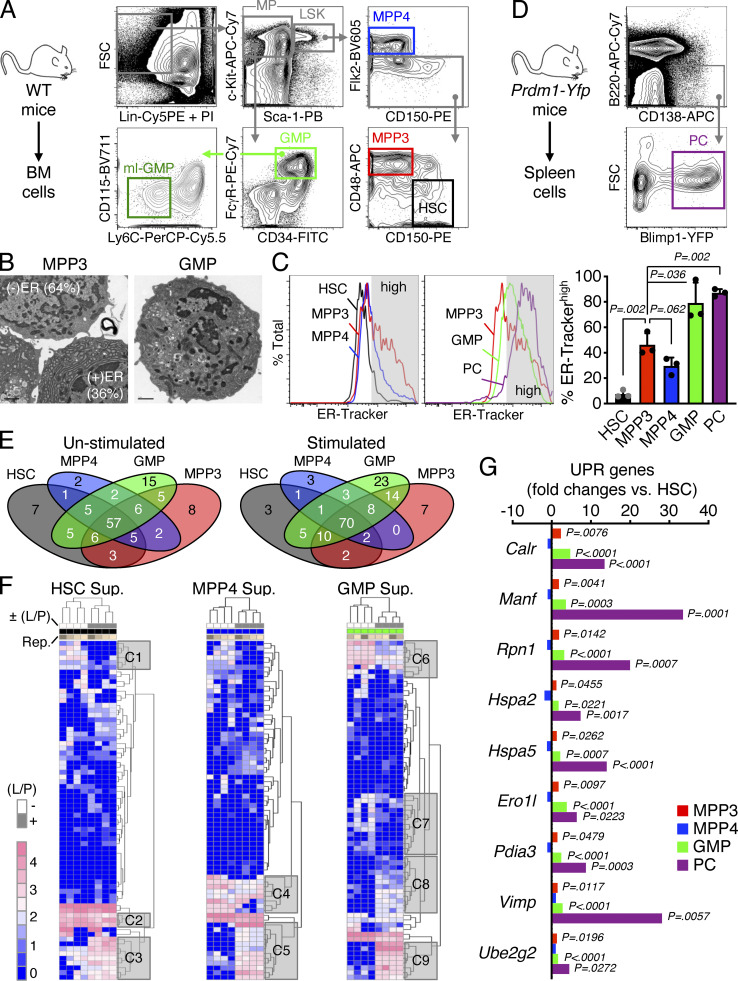
Figure S1.
Secretory activity of HSPCs. (A) Gating strategy used for identifying and isolating BM HSCs and MPPs (MPP3 and MPP4) from the Lin−/Sca-1+/c-Kit+ (LSK) HSPC compartment, as well as GMP and ml-GMP subsets from the Lin−/Sca-1−/c-Kit+ myeloid progenitor (MP) compartment in WT donor mice. (B) Representative example of TEM images used to quantify the percentage of MPP3 with high (+) and low (−) ER volume (n = 69 cells total). Representative TEM image of GMP is shown for comparison to illustrate the differences in morphology. (C) ER-Tracker staining of HSPCs, GMPs, and plasma cells (PC) with representative FACS plots and quantification of ER-Trackerhigh fraction (gray shaded area on histograms). Data are means ± SD (three independent experiments), and significance was assessed by a two-tailed unpaired Student’s t test. (D) Gating strategy used for identifying and isolating splenic plasma cells from Prdm1-Yfp mice. (E and F) Secretory activity of HSPCs and GMPs with (E) overlap in secreted cytokines between populations, and (F) heatmap of unsupervised clustering of secreted cytokines after quantile normalization. Supernatants (Sup.) were collected upon culture of 10,000 cells for 24 h in 150 µl base media ± LPS/Pam3CSK4 (L/P) stimulation; Rep., independent repeats. Uniquely secreted cytokines by each population and representative clusters (C1 to C9) of secreted cytokines changed upon stimulation are provided in Table S1. (G) SABiosciences PCR array of UPR genes in HSPCs, GMPs, and plasma cells (n = 3). Results are expressed as log2 mean fold expression relative to HSCs (set to 0). Significance was assessed by a two-tailed unpaired Student’s t test.