Figure 1.
Design and antigenicity of BG505 SOSIP germline trimer 1.2 (GT1.2)
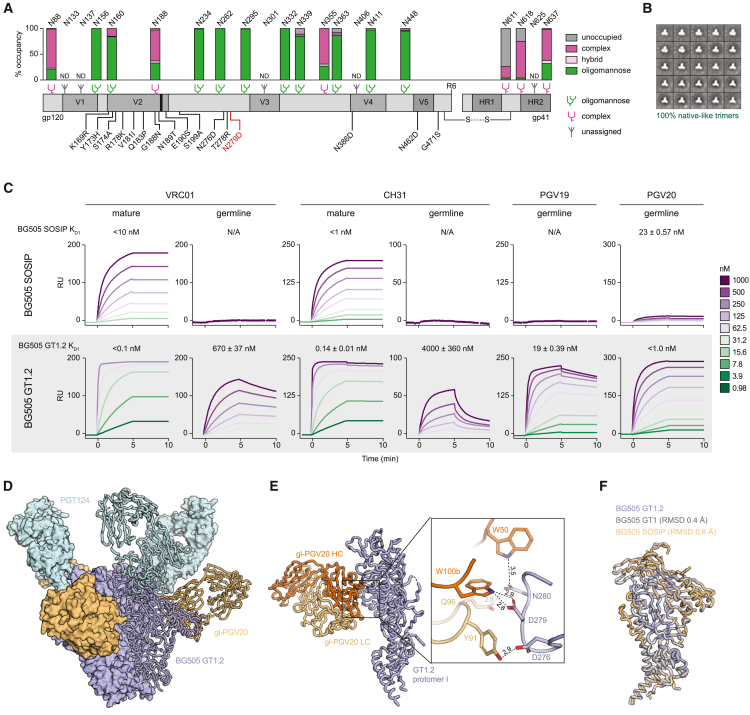
(A) Schematic linear representation of BG505 SOSIP.v4.1 GT1.2 with glycan occupancy data. All amino acid mutations compared with BG505 SOSIP.664 also present in BG505 SOSIPv4.1 GT1 are indicated in black, whereas the N279D substitution defines GT1.2. The glycan icons on top of the linear GT1.2 sequence represent the predominant type of glycan observed at that specific potential N-glycosylation site. ND, not determined.
(B) Negative-stain electron micrograph of soluble GT1.2 trimers.
(C) Surface plasmon resonance sensorgrams showing the specific binding signal in response units (Rus) on the y axes as a function of time during association and dissociation on the x axes for each of the antibody concentrations used (0.98 nM–1,000 nM).
(D) Crystal structure of BG505 SOSIP.v4.1-GT1.2 (blue) trimer in complex with gl-PGV20 (orange) and PGT124 (cyan) Fabs at 3.8 Å resolution.
(E) Side view of the crystal structure of the gl-PGV20 Fab bound to of GT1.2 (blue). (Right) Close-up view of the W100b hydrogen bond interactions of gl-PGV20 with N279 and N280 on GT1.2.
(F) Superimposition of GT1.2 (blue), BG505 SOSIP.664 (orange) (PDB: 5CEZ), and GT1 (gray) (PDB: 5W6D).