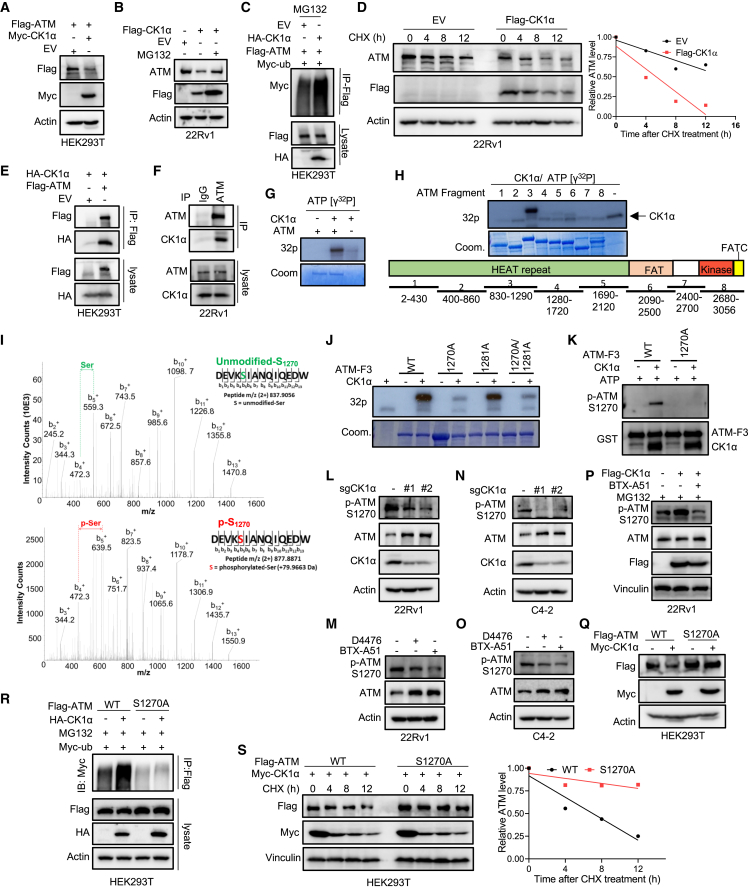
Figure 7.
CK1α regulates ATM stability by phosphorylating ATM
(A) HEK293T cells were co-transfected with FLAG-ATM and Myc-CK1α or EV and harvested for IB.
(B) 22Rv1 cells stably expressing EV or FLAG-CK1α were treated with 20 μM MG132 or DMSO for 6 h and harvested for IB.
(C) HEK293T cells co-transfected with FLAG-ATM, HA-CK1α, and Myc-ubiquitin (ub) were treated with 20 μM MG132 for 12 h and harvested for anti-FLAG immunoprecipitation (IP), followed by IB.
(D) 22Rv1 cells stably expressing EV or FLAG-CK1α were treated with 20 μg mL−1 cycloheximide (CHX) for different times and harvested for IB. The protein abundance of ATM was quantified using Image lab (Bio-Rad).
(E) HEK293T cells were co-transfected with HA-CK1α and FLAG-ATM or EV, and harvested for anti-FLAG IP, followed by IB.
(F) IB analysis of anti-ATM immunoprecipitates and WCL derived from 22Rv1 cells.
(G and H) in vitro kinase assay. After the recombinant human full-length ATM (G) or different fragments (H) were incubated with purified CK1α in the presence of [γ-32P]ATP, the reaction mixtures were resolved by SDS-PAGE followed by autoradiography.
(I) Mass spectrometry (MS) analysis of ATM phosphorylation by CK1α after the in vitro kinase assay of recombinant human ATM fragment 3 with purified CK1α. MS/MS of phosphorylated and intact peptide DEVKSIANQIQEDW for S1270. For clarity, only b+ ions are shown (highlighted in black). The presence of phosphorylation on S1270 was confirmed by the ladders of b3+-b13+ ions in the MS spectrum.
(J) The recombinant human ATM fragment 3 with the indicated mutations were incubated with purified CK1α in the presence of [γ-32P]ATP. The reaction mixtures were resolved by SDS-PAGE followed by autoradiography.
(K) The recombinant human ATM fragment 3 (WT or S1270A) was incubated with purified CK1α in the presence of ATP. The reaction mixtures were resolved by SDS-PAGE followed by IB.
(L and N) IB analysis of WCL derived from 22Rv1 (L) or C4-2 (N) cells expressing sgCtrl or two sgRNAs against CK1α.
(M and O) IB analysis of WCL derived from 22Rv1 (M) or C4-2 (O) cells treated with 20 μM D4476 or 100 nM BTX-A51 for 24 h.
(P) 22Rv1 cells stably expressing EV or FLAG-CK1α were treated with 20 μM MG132 or 100 nM BTX-A51 for 12 h and harvested for IB.
(Q) HEK293T cells were co-transfected with FLAG-ATM (WT or S1270A) and Myc-CK1α, and harvested for IB.
(R) HEK293T cells were co-transfected with FLAG-ATM (WT or S1270), HA-CK1α, and Myc-ub, treated with 20 μM MG132 for 12 h, and harvested for anti-FLAG IP, followed by IB.
(S) HEK293T cells were co-transfected with FLAG-ATM (WT or S1270A) and Myc-CK1α, then treated with 20 μg mL−1 CHX for the indicated times, followed by IB and quantification of protein turnover with Image Lab (Bio-Rad).