Abstract
Background:
In coronavirus disease 2019 (COVID-19) patients, elevated levels of inflammatory cytokines from over stimulation of immune cells have become a concern due to the potential outburst of cytokine storm that damages the tissues and organs, especially the lungs. This leads to the manifestation of COVID-19 symptoms, such as pneumonia, acute respiratory distress syndrome (ARDS), multiple organ failure, and eventually death. Mesenchymal stromal/stem cells (MSCs) are currently one of hopeful approaches in treating COVID-19 considering its anti-inflammatory and immunomodulatory functions. On that account, the number of clinical trials concerning the use of MSCs for COVID-19 has been increasing. However, the number of systematic reviews and meta-analysis that specifically discuss its potential as treatment for the disease is still lacking. Therefore, this review will assess the safety and efficacy of MSC administration in COVID-19 patients.
Objectives:
To pool evidence on the safety and efficacy of MSCs in treating COVID-19 by observing MSC-related adverse effects as well as evaluating its effects in reducing inflammatory response and improving pulmonary function.
Data Sources and Methods:
Following literature search across six databases and one trial register, full-text retrieval, and screening against eligibility criteria, only eight studies were included for data extraction. All eight studies evaluated the use of umbilical cord-derived mesenchymal stromal/stem cell (UC-MSC), infused intravenously. Of these eight studies, six studies were included in meta-analysis on the incidence of mortality, adverse events (AEs), and serious adverse events (SAEs), and the levels of C-reactive protein (CRP) and interleukin (IL)-6. Meta-analysis on pulmonary function was not performed due to insufficient data.
Results:
MSC-treated group showed significantly lower risk of mortality than the control group (p = 0.03). No statistical significance was observed on the incidence of AEs (p = 0.78) and SAEs (p = 0.44), and the levels of CRP (p = 0.06) and IL-6 (p = 0.09).
Conclusion:
MSCs were safe for use, with lower risk of mortality and no association with AEs. Regarding efficacy, descriptive analysis showed indications of improvement on the inflammatory reaction, lung clearance, and oxygenation status despite the lack of statistical significance in meta-analysis of CRP and IL-6. Nevertheless, more studies are needed for affirmation.
Registration:
This systematic review and meta-analysis was registered on the PROSPERO database (no. CRD42022307730).
Keywords: COVID-19, mesenchymal stromal/stem cells, SARS-CoV-2
Introduction
Toward the end of 2019, the whole world faced a novel disease called coronavirus disease 2019 (COVID-19). This infamous disease, which started in Wuhan, China has been declared a global pandemic by the World Health Organization (WHO) on 11 March 2020. 1 Since then, it has been spreading progressively across the world for the last 2 years. As of September 2022, there are roughly 14 million active cases of COVID-19 globally and 6.5 million deaths due to the disease (Worldometers.info, 2022).
COVID-19 is caused by the infection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). It is classified under the Coronaviridae family within the genus Betacoronavirus, along with other well-known viruses, such as Middle East respiratory syndrome coronavirus (MERS-CoV) and SARS-CoV. 2 In comparison with SARS-CoV, SARS-CoV-2 is highly infectious because of its stronger binding of the receptor-binding domain (RBD) of the SARS-CoV-2 with angiotensin-converting enzyme 2 (ACE2).3–5 Since its emergence, the virus continues to evolve and mutate, generating different variants of the virus, with Omicron being the current variant of concern (VOC). 6
Individuals infected by this virus experience symptoms that extend from mild illness with common flu-like symptoms to critical illness with respiratory failure and multi-organ dysfunction, which can lead to death. COVID-19 infection accompanied with cytokine storm is a major threat to the patients as it causes a hyperinflammatory environment that can be fatal. The current treatments, such as antiviral medications and monoclonal antibodies might help in alleviating the symptoms, but most of these treatments might not be sufficient and proven favorable in severely ill COVID-19 patients. Therefore, treatment that can target the major cause of severe progression of COVID-19, namely, cytokine storm, can be beneficial. One of the potential approaches is the use of mesenchymal stromal/stem cells (MSCs).
MSCs are multipotent stem cells with self-renewal ability and differentiation potential into multiple cell lineages, including osteocytes, adipocytes, and chondrocytes.7–10 MSCs have been gaining interest in clinical research, owing to its regenerative and anti-inflammatory effects via immunomodulation of the injured cells.11,12 Notably, numerous state-of-the-art MSC products are emerging rapidly and are predominantly derived from various tissues, such as bone marrow (BM), adipose tissue (AT), and perinatal tissue (PT). BM-MSCs were primarily used clinically until 2008; however, an equal use of MSCs derived from other tissue sources, such as AT and perinatal umbilical cord, has been noticed in the past years. Although MSCs from different sources may share the same characteristics, different MSC products may have different hemocompatibility profiles associated with safety when applied via systemic infusion. Therefore, for efficacious designing and delivery of intravascular MSC therapies, various measures, such as hemocompatibility testing and optimal product delivery, are crucial. 13
Furthermore, MSCs have also been used in cancer research and clinical trials of several inflammatory conditions, including graft versus host disease (GvHD), rheumatoid arthritis, Crohn’s disease, ulcerative colitis and several others.11,12 Basically, MSCs are commonly considered to be used as treatment for inflammatory diseases. Therefore, MSCs can theoretically be used for COVID-19 treatment, as the disease progression to its severe form is associated with an aberrant immune response that causes hyperinflammatory state and cytokine storm. Moreover, its regenerative function can help in recovering injured alveolar epithelial cells.
On that account, the number of clinical trials concerning the use of MSCs for COVID-19 therapy has been increasing. However, the numbers of systematic reviews that specifically discuss its potential as treatment for the disease are still lacking. This review will assess the safety and efficacy of MSCs in resolving complications in COVID-19 patients.
Methods
Systematic review and meta-analysis
Study registration
This systematic review was registered under PROSPERO, international prospective register of systematic reviews with registration ID of CRD42022307730 (https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022307730). The protocol was planned prior to registration according to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) 2020 checklist. 14
Eligibility criteria
The eligibility criteria were defined according to the PICOS (population, intervention, comparison, outcomes and study) format. 15
Population
The target population in this systematic review was COVID-19 patients receiving MSCs treatment. The included patients were at least 18 years and above (adult and older adult) regardless of gender. The SARS-CoV-2 infection in each patient was laboratory confirmed and only patients with disease severity ranging from moderate, severe to critical COVID-19 were included. Non-human studies were excluded.
Intervention
The intervention for the experimental group was the administration of human-derived non-modified MSCs from BM, PTs (e.g. placenta, umbilical cord), Wharton’s jelly, and ATs. Studies that used MSCs derived from embryonic stem cells (ESCs) and induced pluripotent stem cells (IPSC) were excluded. Studies that used modified or genetically modified MSCs were also excluded. On the other hand, standard treatments or placebo were given to the control group.
Comparison
COVID-19 patients who did not receive MSCs treatment were observed in comparison with the target population.
Outcomes
The outcomes were divided into three categories: (1) occurrence of mortality and adverse events (AEs), (2) reduction in inflammatory reaction based on the level of inflammatory markers, and (3) improvement in pulmonary function that was observed from the ’patient’s oxygenation and/or pulmonary imaging.
Study design
Randomized controlled trial (RCT) with parallel and cross-over designs and case reports were included. The included studies must be published in English language with original and complete data irrespective of publication status (e.g. peer-reviewed, preprint). Clinical reviews, editorials, and letters were excluded.
Information sources and search strategy
Article searches were conducted across six databases and one trial register: (1) PubMed, (2) ScienceDirect, (3) EBSCOhost, (4) Google Scholar, (5) The Cochrane Library, (6) bioRxiv, and (7) Clinical Trials.gov. The search terms and limitations are listed in Table 1.
Table 1.
Search terms and limitations used in article searches.
| Databases | Search terms and limitations |
|---|---|
| PubMed Last searched: 23 March 2022 |
Search terms: (Mesenchymal stem cells OR Mesenchymal stromal cells OR MSC) AND (COVID-19 OR SARS-CoV-2) Filter: The search results were filtered for clinical trials and randomized controlled trials only |
| ScienceDirect Last searched: 23 March 2022 |
Search terms: (Mesenchymal stem cells OR Mesenchymal stromal cells OR MSC) AND (COVID-19 OR SARS-CoV-2) AND (cytokine storm OR cytokine release syndrome) Filters: • The year of publication was set from 2020 to 2022 to remove irrelevant articles • The results were filtered to generate research articles and case reports only |
| EBSCOhost Last searched: 24 March 2022 |
Search terms: Mesenchymal stem cells OR Mesenchymal stromal cells OR MSC) AND (COVID-19 OR SARS-CoV-2) AND (cytokine storm OR cytokine release syndrome) Filters: • Searches by Boolean/Phrase was applied • Results were filtered for full text English articles • The terms were searched within full text of the articles • Search for equivalent subjects was applied • The year of publication was set from 2020 to 2022 to remove irrelevant articles • The source type was filtered to academic journals only |
| Google Scholar Last searched: 25 March 2022 |
Search terms: (Mesenchymal stem cells OR Mesenchymal stromal cells OR MSC) AND (COVID-19 OR SARS-CoV-2) AND cytokine storm Filter: • The year of publication was set from 2020 to 2022 to remove irrelevant articles |
| The Cochrane Library Last searched: 23 March 2022 |
Search terms: (Mesenchymal stem cells OR Mesenchymal stromal cells OR MSC) AND (COVID-19 OR SARS-CoV-2) *No filter applied |
| bioRxiv Last searched: 23 March 2022 |
Search terms: (Mesenchymal stem cells OR Mesenchymal stromal cells OR MSC) AND (COVID-19 OR SARS-CoV-2) AND (cytokine storm OR cytokine release syndrome) Filters: • The year of publication was set from 2020 to 2022 to remove irrelevant articles • The search was conducted across both bioRxiv and medRxiv |
| ClinicalTrials.gov Last searched: 24 March 2022 |
Search terms: (Mesenchymal stem cells OR Mesenchymal stromal cells OR MSC) AND (COVID-19 OR SARS-CoV-2) Filter: The results were filtered for completed studies only |
SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Selection process
The search results were imported to Zotero to screen for duplicates. Subsequently, the remaining articles were exported to Microsoft Excel Spreadsheet Software for title and abstract screening by the reviewers. Next, full-text articles were retrieved prior to full-text screening. Full-text screening was conducted independently by two reviewers [Hannah Taufiq (HT) and Syahnaz Rusli (SR)]. Any disagreements were resolved through discussion between the two reviewers or decision-making by the third and fourth reviewers [Syahril Abdullah (SA) and Kamal Shaik Fakiruddin (KSF)]. The selection process was conducted according to the PRISMA 2020 flow diagram for searches of databases and registers (Figure 1).
Figure 1.
PRISMA 2020 flow diagram for systematic reviews. 14
Data extraction
The data from the included studies were extracted independently by the first reviewer (HT) and cross-checked for accuracy by two reviewers (SA and KSF). The data were extracted into five tables:
Table 2: Patient characteristics.
Table 3: Study characteristics.
Table 4: Incidence of mortality and adverse events.
Table 5: Effect of MSCs on inflammatory response.
Table 6: Effect of MSCs on pulmonary function.
Table 2.
The eligibility criteria and characteristics of patients included in various studies.
| Sources | Participants | ||
|---|---|---|---|
| Age (years) |
Number (n) | Morbidity definition | |
| Adas et al.
21
Turkey |
40–60 | Total: 30 Male: 19 Female: 11 |
• Confirmed COVID-19-related severe ARDS • Stages: Moderate to critical illness |
| Dilogo et al.
22
Indonesia |
18–95 | Total: 40 Male: 30 Female: 10 |
• RT-PCR confirmed COVID-19 cases • Stage: critical illness • Presence of pneumonia on chest X-ray and/or GGO on CT images |
| Lanzoni et al.
19
United States |
⩾ 18 | Total: 24 Male: 13 Female: 11 |
• SpO2 ⩽ 94% / required supplemental
oxygen • PaO2/FiO2-ratio < 300 mmHg • Chest radiograph demonstrated bilateral infiltrates/CT images showed ground-glass opacities |
| Liang et al.
23
China |
65 | Total: 1 Female: 1 |
• Real time RT-PCR confirmed COVID-19 case • Stage: critical illness • Severe pneumonia (mixed type) • ARDS • Multiple organ injury involving liver, blood, and respiratory system |
| Monsel et al.
20
France |
> 18 | Total: 45 Male: 37 Female: 8 |
• RT-PCR confirmed COVID-19 cases • Meet Berlin criteria-defined ARDS for < 96 h |
| Rebelatto et al.
24
Brazil |
> 18 | Total: 17 Male: 12 Female: 5 |
• RT-PCR confirmed COVID-19 cases • Stage: critical illness (WHO ordinal scale scores 6 and 7) • PaO2/FiO2 ratio ⩽ 300 mmHg |
| Shu et al.
25
China |
18–90 | Total: 41 Male: 24 Female: 17 |
• Real-time RT-PCR confirmed COVID-19 cases • Stage: severe illness • CT images show the presence of pneumonia • Respiratory distress with RR of ⩾ 30 times/min • SpO2 ⩽ 93% at rest • PaO2/FiO2-ratio ⩽ 300 mmHg |
| Zhu et al.
26
China |
48 | Total: 1 Male: 1 |
• Nucleic acid test confirmed COVID-19 case • Stage: critical illness • ARDS • Multiple organ injury involving hepatic and respiratory systems |
ARDS, acute respiratory distress syndrome; CT, computerized tomography; RR, risk ratio; RT-PCR, reverse transcription polymerase chain reaction; WHO, World Health Organization; GGO, ground-glass opacity; CT, computerized tomography.
Table 3.
Study characteristics comprising the design and details of MSC treatment.
| Sources | Study types | Participants (n) | Mesenchymal stromal/stem cells | ||||
|---|---|---|---|---|---|---|---|
| Experimental group | Control group | Sources | Passage | Dose | Delivery method | ||
| Adas et al. 21 | Randomized, standard treatment-controlled trial, three parallel armed (two control arms) | Group 3 (critical illness): 10 | Group 1 (moderate illness): 10 Group 2 (critical illness): 10 |
Wharton’s Jelly | 4 | 3 × 106 cells/kg in 150 ml of 0.9% NaCl (three infusions) | Intravenous infusion |
| Dilogo et al. 22 | Multicentered, double-blind, randomized, placebo- controlled trial | 20 | 20 | Umbilical cord | 5 or 6 | 1 × 106cells/kg in 100 ml of 0.9% NaCl (single infusion) | Intravenous infusion |
| Lanzoni et al. 19 | Phase I/IIa, double-blind, randomized, placebo-controlled trial | 12 | 12 | Umbilical cord | N/A | 100 ± 20 × 106 cells/infusion in 50 ml vehicle solution containing HSA and heparin (two infusions) | Intravenous infusion |
| Liang et al. 23 | Case report | 1 | 0 | Umbilical cord | 5 | 5 × 107cells/infusion in 0.9% NaCl with 5% human albumin (three infusions) | Intravenous infusion |
| Monsel et al. 20 | Multicentered, double-blind, randomized, placebo- controlled trial | 21 | 24 | Umbilical cord | 4 | 1 × 106cells/kg in 150 ml of 0.9% NaCl with 0.5% albumin (three infusions) | Intravenous infusion |
| Rebelatto et al. 24 | Phase I/II, prospective, single-centered, randomized, double-blind, placebo-controlled clinical trial | 11 | 6 | Umbilical cord | 3–5 | 5 × 105 cells/kg in 30 mL of vehicle solution containing saline solution, 5% anticoagulant citrate dextrose (ACD), and 20% albumin (three infusions) | Intravenous infusion |
| Shu et al. 25 | Single-centered open-label, individually randomized, standard treatment-controlled trial | 12 | 29 | Umbilical cord | 3–5 | 2 × 106 cells/kg in 100 ml of normal saline (single infusion) | Intravenous infusion |
| Zhu et al. 26 | Case report | 1 | 0 | Umbilical cord | N/A | 1 × 106cells/kg in 100 ml of 0.9% NaCl (single infusion) | Intravenous infusion |
MSC, mesenchymal stromal/stem cell; HAS, human serum albumin; N/A, not available.
Table 4.
Incidence of mortality and adverse events in MSC-treated and control groups.
| Sources | MSC-treated | Control | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number of death events | Number of patients with AEs | Number of patients with SAEs | Total | Mortality rate (%) | Number of death events | Number of patients with AEs | Number of patients with SAEs | Total | Mortality rate (%) | |
| Adas et al.
21
Turkey |
3 | N/A | N/A | 10 | 30 | 6 | N/A | N/A | 10 | 60 |
| Dilogo et al.
22
Indonesia |
10 | N/A | N/A | 20 | 50 | 16 | N/A | N/A | 20 | 80 |
| Lanzoni et al.
19
United States |
2 | 8 | 2 | 12 | 16.67 | 7 | 11 | 8 | 12 | 58.33 |
| Liang et al.
23
China *Case report |
0 | 0 | 0 | 1 | 0 | N/A | N/A | N/A | N/A | N/A |
| Monsel et al.
20
France |
5 | 18 | 6 | 21 | 23.81 | 4 | 18 | 6 | 24 | 16.67 |
| Rebelatto et al.
24
Brazil |
5 | N/A | N/A | 11 | 45.45 | 1 | N/A | N/A | 6 | 16.67 |
| Shu et al.
25
China |
0 | N/A | N/A | 12 | 0 | 3 | N/A | N/A | 29 | 10.34 |
| Zhu et al.
26
China *Case report |
0 | 0 | 0 | 1 | 0 | N/A | N/A | N/A | N/A | N/A |
AE, adverse event; N/A, not available; MSC, mesenchymal stromal/stem cell; SAE, serious adverse event.
Table 5.
Effect of MSCs on inflammatory response in COVID-19 patients in eight included studies.
| Sources | CRP (M ± SD) | IL-6 (M ± SD) | Additional article remarks | ||
|---|---|---|---|---|---|
| MSC-treated | Control | MSC-treated | Control | ||
| Adas et al.
21
Turkey |
99.2 ± 90.8 mg/l *7 days after infusion |
139.6 ± 72.05 mg/l *7 days after infusion |
91.3 ± 82.1 pg/ml *7 days after infusion |
117.3 ± 44.2 pg/ml *7 days after infusion |
COVID-19 inflammatory markers: • On day 7 after infusion in critically ill patients, the levels of PCT and CRP in the treatment group was significantly lower (p < 0.05) than the control group Pro-inflammatory cytokines: • Levels of IL-6 were significantly lower in MSC treated critical cases (p < 0.05) compared with the control group • Levels of TNF-α showed no statistical significance (p > .05) between groups • Levels of IL-2 showed a steady decrease in the treatment group Anti-inflammatory cytokines: • On day 7 after infusion in critically ill patients, the levels of IL-10 were significantly higher in the treatment group (p < 0.05) compared with the control group |
| Dilogo et al.
22
Indonesia |
Value not stated in the paper | Value not stated in the paper | 12.46 pg/ml | 25.67 pg/ml | COVID-19 inflammatory markers: • As stated in the paper, no significant difference was observed in the levels of PCT and CRP between treatment and control groups during 15 days of observation Pro-inflammatory cytokines: • By day 7 after infusion of MSCs, IL-6 levels decreased in the treatment group but increased in the control group. Significant decrease in IL-6 was observed in recovered patients after infusion (p = 0.023) Anti-inflammatory cytokines: • At day 7, level of IL-10 increased in the treatment group but decreased in the control group with no significant differences. |
| Lanzoni et al.
19
United States |
N/A | N/A | Value not stated in the paper | Value not stated in the paper | Pro-inflammatory cytokines: • As stated in the paper, comparison between groups showed significant decrease in the levels of IL-6 and TNF- α only in the treatment group (p < 0.05) by day 6 • The level of IL-2 also decreased by day 6 in the treatment group compared with the control group with value close to statistical significance (p = 0.051) • From days 0 to 6, longitudinal analysis demonstrated significant decrease of the inflammatory cytokines only in the treatment group |
| Liang et al.
23
China *Case report |
N/A | N/A | N/A | N/A | COVID-19 inflammatory markers: • On day 16 after disease onset (second infusion of MSC), levels of CRP showed a rapid decrease from 27.9–11.93 mg/l. *Normal range: 0–3 mg/l • On day 16 after disease onset (second infusion of MSC), levels of PCT showed a rapid decrease from 0.153–0.047 ng/ml *Normal range: 0–0.05 ng/ml |
| Monsel et al.
20
France |
N/A | N/A | N/A | N/A | Pro-inflammatory cytokines: • Levels of TNF- α decreased significantly (p < 0.05) in the treatment group (D7 and D14) but the decline was observed later than the decline of pro-inflammatory cytokines in the control group (D4 and D7). Anti-inflammatory cytokines: • Levels of IL-10 decreased significantly (p < 0.05) in the treatment group (D7 and D14), but the decline was observed later than the decline of pro-inflammatory cytokines in the control group (D4 and D7). |
| Rebelatto et al.
24
Brazil |
0.88 ± 1.22 mg/dl *4 months after infusion |
4 ± 5 mg/dl *4 months after infusion |
3.70 ± 2.89 pg/ml *4 months after infusion |
100.97 ± 173.60 pg/ml *4 months after infusion |
COVID-19 inflammatory markers: • Levels of CRP significantly decreased between baseline and month 2 (p = 0.01), and baseline and month 4 (p = 0.01) in the treatment group while no differences were observed in the control group Pro-inflammatory cytokines: • Levels of IL-6 significantly decreased between baseline and day 14 (p = 0.02), baseline and month 2 (p = 0.01) and baseline and month 4 (p = 0.01) in the treatment group while the levels of IL-6 remained high in the control group between time points • Comparison between groups showed higher levels of IL-6 in the treatment group up until day 4 after infusion, where significant decrease and increase were observed in treatment and control groups, respectively (p = 0.01). • No differences were observed in the levels of IL-2 and TNF-α in both groups at each time point Anti-inflammatory cytokines: • IL-10 showed a higher level of expression in the MSC-treated group compared with the control group throughout all time points |
| Shu et al.
25
China |
Value not stated in the paper | Value not stated in the paper | Value not stated in the paper | Value not stated in the paper | COVID-19 inflammatory markers: • As stated in the paper, levels of CRP significantly decreased in the treatment group compared with the control group (p < 0.001) starting from day 3 of MSC infusion Pro-inflammatory cytokines: • As stated in the paper, levels of IL-6 significantly decreased in the treatment group compared with the control group (p < 0.01) starting from day 3 of MSC infusion |
| Zhu et al.
26
China *Case report |
N/A | N/A | N/A | N/A | COVID-19 inflammatory markers: • Levels of CRP showed a steady decrease from 150 mg/l before infusion to 1.28 mg/L 13 days after infusion *Normal range: 0–3 mg/l • Levels of PCT returned to normal level from 0.766 ng/ml before infusion to 0.024 ng/ml 13 days after infusion *Normal range: < 0.046 ng/ml |
CRP, C-reactive protein; IL-6, interleukin-6; MSC, mesenchymal stromal/stem cell; PCT, procalcitonin; TNF-α, tumor necrosis factor-alpha; N/A, not available.
Table 6.
Effect of MSCs on pulmonary function in eight included studies.
| Sources | Pulmonary function and imaging |
|---|---|
| Adas et al.
21
Turkey |
Oxygenation status: • PaO2/FiO ratio was lower in the treatment group during the treatment with no significant differences between groups • Duration of ventilator use in the treatment group was significantly lower than the control group after day 7 following infusion |
| Dilogo et al.
22
Indonesia |
N/A |
| Lanzoni et al.
19
United States |
N/A |
| Liang et al.
23
China *Case report |
CT features: • On days 8 and 13 after first infusion, CT images showed reduction of GGO |
| Monsel et al.
20
France |
Oxygenation
status: • PaO2/FiO2-ratio changes between D0 and D7 did not show any significant differences between treatment and control group • PaO2/FiO ratio showed significant increase within the treatment group from days 0 to 7 (p = 0.03) for alive patients remaining after 7 days • PaO2/FiO2 ratio within the control group did not show significant differences (p = 0.70) from days 0 to 7 |
| Rebelatto et al.
24
Brazil |
CT features: • CT images showed higher lung clearance in the treatment group compared with the control group based on the degree of opacification with statistical significance between baseline and month 4 (p = 0.01), and day 14 and month 4 (p = 0.01) |
| Shu et al.
25
China |
Oxygenation status: • On day 7, 66.67% of patients in the treatment group and 10.34% in the control group did not require supplemental oxygen • Time taken for oxygenation index return to normal was significantly shorter in the treatment group (p < 0.001) when compared with the control group CT features: • Two weeks after treatment, CT scores (p = 00.017), the number of lobes involved (p < 0.001), GGO (p = 0.0407), and consolidation (p = 0.0306) were significantly improved in the treatment group compared with the control group |
| Zhu et al.
26
China *Case report |
Oxygenation status: • On day 2 after infusion, SpO2 returned to normal level at 95% and above CT features: • On day 7 after infusion, CT images showed reduction of GGO and pneumonia infiltration |
CT, computerized tomography; GGO, ground-glass opacity; MSC, mesenchymal stromal/stem cell.
Quality assessment
The risk of bias assessment for RCTs was performed using the Cochrane risk-of-bias 2 (RoB-2) tool for randomized trials. 16 It assessed five domains:
Domain 1 (D1). Bias arising from the randomization process
Domain 2 (D2). Bias due to deviations from intended interventions
Domain 3 (D3). Bias due to missing outcome data
Domain 4 (D4). Bias in measurement of the outcome
Domain 5 (D5). Bias in selection of the reported result
Under these domains, a series of questions were answered with ‘Yes’, ‘Probably yes’, ‘No information’, ‘Probably no’, or ‘No’ for every included study. The risk of bias for each domain was determined based on the combination of these answers. Then, the overall risk of bias for each study was determined as low risk, some concerns, or high risk. Following this, the assessment was visualized using robvis tool. 17 Quality assessment for RCTs was conducted on the incidence of mortality, Aes, and serious adverse effects (SAEs), as well as the levels of C-reactive protein (CRP) and interleukin (IL)-6. The effect of interest was ‘intention to treat’.
On the other hand, case reports included were assessed using JBI critical appraisal checklist for case reports. 18 The checklist consists of eight questions related to the case report, such as the patient’s condition, pre-, and post–treatments as well as procedures throughout the study. Subsequently, overall appraisal is determined to decide the inclusion or exclusion of the case report. Both quality assessment for RCTs and case reports were conducted by two reviewers (HT and SR) and cross-checked by another two reviewers (SA and KSF).
Data analysis and synthesis
Statistical analyses were performed using Review Manager (REVMAN) 5.4 to generate forest plots. The pooled effect was measured as risk ratio (RR) and odds ratio (OR) for dichotomous data and standardized mean difference (SMD) for continuous data with 95% confidence interval (CI). In this review, dichotomous data are the mortality incidence and occurrence of Aes and SAEs. On the other hand, continuous data are the level of inflammatory cytokines. Meta-analysis for pulmonary function was not performed due to insufficient data to enable analysis. Fixed-effect model was used when the heterogeneity level was not significant (I² ⩽ 50%), whereas the random-effect model was used when the heterogeneity level was significant (I² > 50%).
Publication bias
The publication bias was conducted using Review Manager (REVMAN) 5.4. Funnel plots were generated and analyzed to detect the presence of publication bias on every outcome that was analyzed by the software. Presence of publication bias is presented as asymmetrical funnel plot whereas absence of publication bias is presented as symmetrical funnel plot.
Results
Literature search
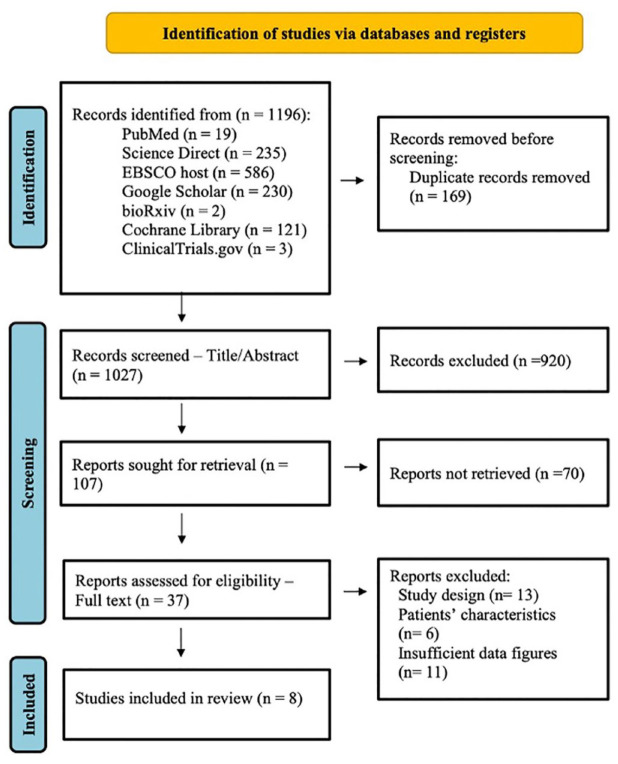
Literature search across six databases and one trial register had resulted in 1196 articles. The keywords that were used to run the search are mesenchymal stem cells, mesenchymal stromal cells, MSC, COVID-19, SARS-CoV-2, cytokine storm, and cytokine release syndrome. These keywords were used with Boolean operators, OR and AND. The number of articles identified from each database and register are stated in Figure 2. The 1196 articles were then screened for duplicates. The screening removed 169 duplicates and reduced the articles number to 1027. Subsequently, the articles were screened for title and abstract. Accordingly, 920 irrelevant articles were removed leaving 107 articles to be sought for full-text retrieval. Full text was retrieved through request from authors and database search.
Figure 2.
PRISMA 2020 flow diagram. 14
Of the 107 articles, 70 articles were removed due to failure in full-text retrieval. The remaining 37 articles were included for full-text screening against the eligibility criteria. After full-text screening, eight articles were finalized and selected for data extraction. The other 29 articles were excluded due to study design, patients’ characteristics, and incomplete data figures. Thirteen studies did not meet the study design because of non-randomized arrangement of participants and absence of control. Six studies were excluded for incompatible patients’ characteristics (i.e. age) or incomplete patients’ data. Eleven (11) studies were excluded due to insufficient or missing data figures. One of the studies was excluded for two reasons, which are unmet study design and missing data.
Data extraction
Following full-text screening, eight studies had been selected for data extraction. The studies originated from various countries, including Turkey (n = 1), Indonesia (n = 1), the United States (n = 1), China (n = 3), France (n = 1), and Brazil (n = 1). All studies were published within the last 2 years, ranging from 2020 to 2022. The extracted data from all studies were presented in five tables. Table 2 summarized the characteristics of patients who participate in each study. All patients were ⩾ 18 years old with laboratory-confirmed COVID-19 infection. The stages of disease that were included in this review are moderate to critical illness. In studies that did not state the disease stages, the baseline characteristics or the eligibility criteria were to follow the clinical spectrum for moderate to critical COVID-19 according to National Institutes of Health (NIH), US Department of Health and Human Services.19,20 The eligibility criteria provided by Lanzoni et al. 19 and Monsel et al. 20 (Table 2) met the NIH clinical spectrum for severe COVID-19 and critical COVID-19, respectively.
Furthermore, Table 3 summarizes the characteristics of each study included in this systematic review that comprises study design and details of treatment used. Six of eight studies are RCTs while the remaining two are clinical case reports of a patient. All the RCTs used 1:1 ratio during randomization of participants into treatment and control groups except Rebelatto et al. 24 that randomized at a 1:2 ratio, while Shu et al. 25 did not state any randomization ratio. The MSCs used in all studies were from umbilical cord that was harvested at passage 3–6. Different studies used different doses but all of them were infused intravenously into the patients.
Tables 4–6 highlighted the outcomes of the study, which are the occurrence of mortality and AEs, alleviation of inflammatory responses, and improvement in pulmonary function, respectively. Table 4 summarized the number of patients with the incidence of AEs, SAEs, and deaths. From the table, lower mortality rate was observed in MSC-treated groups compared with control groups in all studies except Monsel et al. 20 and Rebelatto et al. 24 As for the incidence of AEs and SAEs, Lanzoni et al. 19 reported lower numbers of patients with the events in the MSC-treated group than the control group. However, Monsel et al. 20 reported the same number of patients with AEs and SAEs in both groups. The extracted outcome data are further discussed in the ‘Discussion’ section.
Meanwhile, in Table 5, the inflammatory responses were observed based on the level of COVID-19 inflammatory markers [e.g. CRP and procalcitonin (PCT)], pro-inflammatory cytokines [e.g. IL-6, IL-2, and tumor necrosis factor alpha (TNF-α)], and anti-inflammatory cytokines (e.g. IL-10). On top of that, the mean and standard deviation of CRP and IL-6 at the endpoint were particularly recorded for meta-analysis. Based on the table, all studies that measured CRP and PCT showed a notable decrease of the cytokines in MSC-treated groups compared with control groups except for Dilogo et al., 22 which observed no significant difference between the two groups. The level of IL-6 also showed significant decrease in MSC-treated groups compared with control in all applicable studies.19,21,22,24,25 In addition, studies by Adas et al. 21 and Dilogo et al. 22 reported increased levels of IL-10, an anti-inflammatory cytokine. Furthermore, Rebelatto et al. 24 show a trend of higher IL-10 expression in the MSC-treated group compared with the control group. Other cytokines, such as IL-2, IL-12, and TNF-α, varied in concentration across different studies (Table 5).
Finally, in Table 6, the pulmonary function was observed based on the oxygenation status and computerized tomography (CT) features of the patients. Based on studies by Adas et al. 21 and Monsel et al., 20 the PaO2/FiO2 ratio showed no significant differences between MSC-treated and control groups, but Monsel et al. reported significant increase of the ratio between days 0 and 7 following infusion. In respect of CT features, all applicable studies reported improvement in the lung clearance, pneumonia infiltration, the number of lobes involved, ground-glass opacity (GGO), consolidation, and CT scores.23–26
Quality assessment
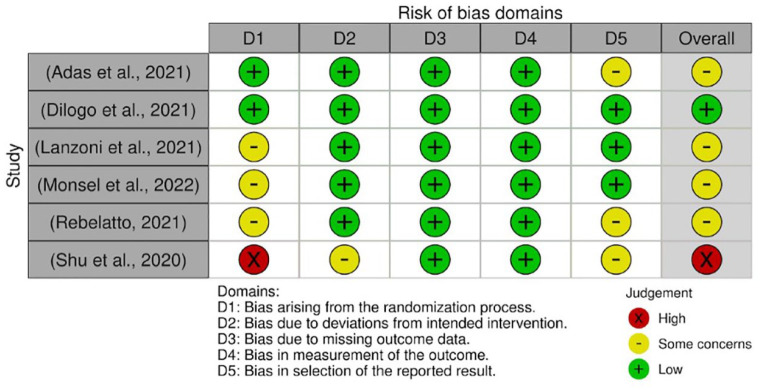
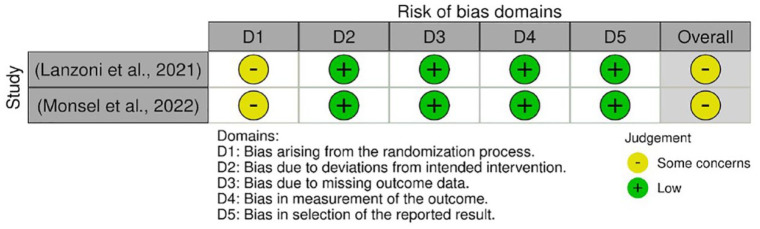
Quality assessment for RCTs
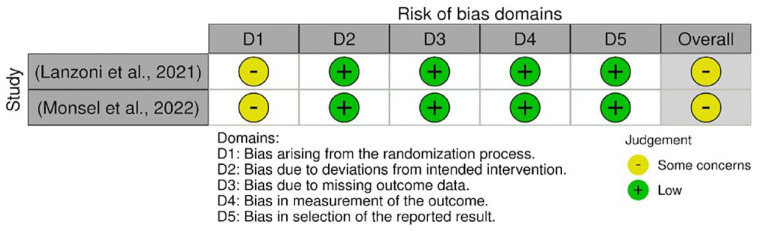
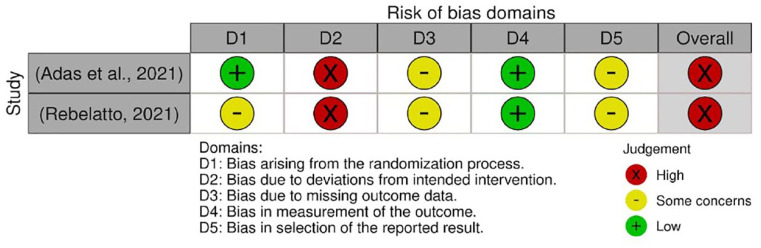
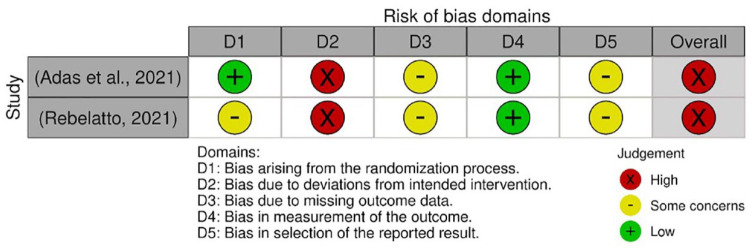
The quality assessments were conducted on every outcome analyzed in the meta-analysis. The results from quality assessment through the Cochrane RoB-2 tool are shown in Figures 3–7. Most of the studies were judged as ‘some concerns’ due to potential bias from the randomization process. This is because baseline imbalances were observed in those studies. In addition, Shu et al. 25 were judged as ‘high risk’ as the trial was conducted in an open-label procedure. As for the levels of CRP and IL-6, both studies were judged as ‘high risk’ due to the possibility of missing outcome data that might influence the results. Moreover, both studies did not state the intended principal analysis (e.g. intention-to-treat analysis and modified intention-to-treat) or any analysis conducted to correct the bias. The summary on judgment and reasons for judgments are stated in detail in the Supplemental Material (Appendix A).
Figure 3.
Quality assessment on included studies with respect to the mortality incidence.
Figure 4.
Quality assessment on included studies with respect to the incidence of AEs.
Figure 5.
Quality assessment on included studies with respect to the incidence of SAEs.
Figure 6.
Quality assessment on included studies with respect to the level of CRP.
Figure 7.
Quality assessment on included studies with respect to the level of IL-6.
Quality assessment for case reports
Quality assessment for case reports was conducted through Joanna Briggs Institute (JBI) critical appraisal checklist for case reports. 18 The two case reports were included in the review as both met most of the checklists for a good case report.23,26 The checklists for the two case reports are provided in the Supplemental Material (Appendix B).
Data synthesis
Six RCTs were included for meta-analysis provided that the studies have relevant data for analysis. The two case reports were excluded. For Adas et al., 21 results from group 1 were excluded to maintain randomization of the study. From the meta-analysis, five forest plots (Figures 8–10) were generated using REVMAN 5.4 to evaluate pooled effects from all studies on the safety and efficacy of MSCs in COVID-19 patients. In the forest plot, the point effect estimates for individual studies are presented as a box (blue) that is in line with each study. This box also reflects the size of the study. Bigger box is associated with more study participants. In addition, the horizontal line that can be seen with the box represents the 95% CI of the effect estimate for each study. The width of the lines represents the boundaries of the CI, which depends on the precision of each study result. Larger studies with a higher number of participants usually give more precise effect estimates. Hence, larger studies have narrower CI compared with smaller studies. Ensuing the individual study results, pooled effect estimates from all studies are presented as the black diamond with its width as the 95% CI. The values for individual and pooled effect estimates along with the range of CI can also be seen in the forest plot. Finally, statistical significance of the meta-analysis can be obtained at the bottom of the forest plot.
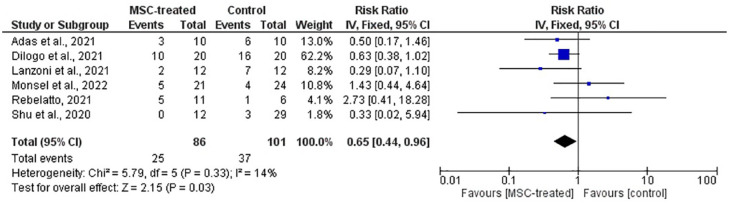
Figure 8.
Forest plot of meta-analysis on the incidence of mortality from six RCTs. The blue box denotes the effect estimate for each study, whereas the black diamond denotes the pooled effect estimates from all studies. Events column stands for number of death cases reported while total column stands for the number of participants in the group.
Figure 9.
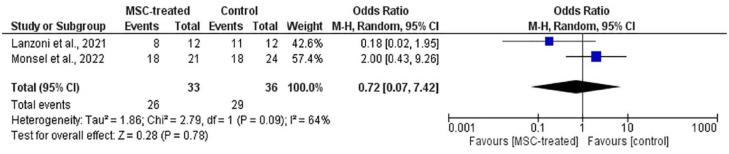
Forest plot of meta-analysis on the incidence of AEs from two RCTs. The blue box denotes the effect estimate for each study, whereas the black diamond denotes the pooled effect estimates from all studies. Events column stands for number of participants with AEs while total column stands for the number of participants in the group.
Figure 10.
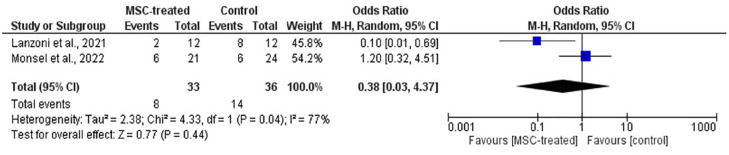
Forest plot of meta-analysis on the incidence of SAEs from two RCTs. The blue box denotes the effect estimate for each study, whereas the black diamond denotes the pooled effect estimates from all studies. Events column stands for number of participants with SAEs while total column stands for the number of participants in the group.
Meta-analysis on safety of MSCs as treatment for COVID-19
The incidences of mortality, AEs, and SAEs in all studies were observed to analyze the safety of MSCs. Based on Figure 8, the overall RR on the incidence of mortality is 0.65 (CI = 0.44, 0.96) in favor of the MSC-treated group with the presence of statistical significance (p = 0.03). Hence, the risk of mortality is lower in the MSC-treated group in comparison with the control group.
In addition, Figures 9 and 10 show no statistical significance on the incidence of AEs [p = 0.78; OR (CI) = 0.72 (0.07, 7.42)] and SAEs [p = 0.44; OR (CI) = 0.38 (0.33, 4.37)]. This indicates that the infusion of MSCs has no association with the occurrence of AEs and SAEs among the patients. From Figures 9 and 10, high heterogeneity was observed in meta-analysis on the incidence of AEs (I2 = 64%) and SAEs (I2 = 77%). In dealing with this issue, a random effects model was used. Subgroup analysis was not applicable due to a small number of studies.
Meta-analysis on efficacy of MSCs as treatment for COVID-19
The efficacy of MSCs in this review was observed based on the level of inflammatory markers and pulmonary function in the patients. However, meta-analysis was not performed on pulmonary function due to insufficient data. As for the inflammatory markers, in order only CRP and IL-6 were included in meta-analysis to standardize the results from all studies. In the work of Rebelatto et al., 24 one participant was excluded due to loss to follow-up appointment after cell infusion. Hence, the total participants were reduced to 10 from 11.
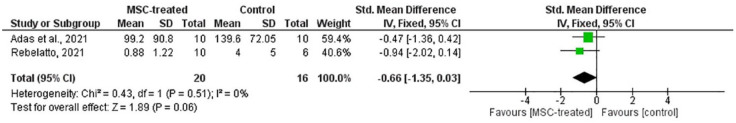
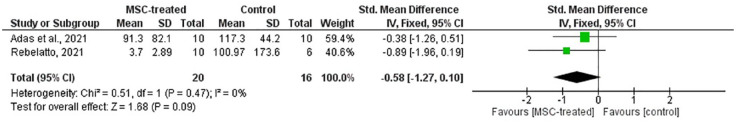
Based on Figures 11 and 12, SMD of CRP [p = 0.06; SMD (CI) = –0.66 (–1.35, 0.03)] and IL-6 [p = 0.09; SMD (CI) = –0.58 (–1.27, 0.10)] showed no statistical significance between MSC-treated and control groups. This suggests that the level of CRP and IL-6 in both treatment and control groups were not significantly different. Therefore, the ability of MSC in reducing inflammatory response cannot be determined.
Figure 11.
Forest plot of meta-analysis on the level of CRP from two RCTs. The green box denotes the effect estimate for each study, whereas the black diamond denotes the pooled effect estimates from all studies. Mean and standard deviation of CRP level along with total participants in the group were listed under the column for both MSC-treated and control groups.
Figure 12.
Forest plot of meta-analysis on the level of IL-6 from two RCTs. The green box denotes the effect estimate for each study whereas the black diamond denotes the pooled effect estimates from all studies. Mean and standard deviation of CRP level along with total participants in the group were listed under the column for both MSC-treated and control groups.
Publication bias
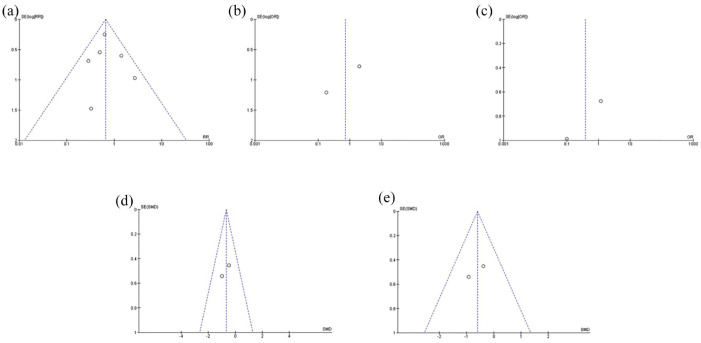
Five funnel plots were generated using REVMAN 5.4 to assess the publication bias among included studies on every outcome. Based on Figure 13, all funnel plots were seen to be symmetrical suggesting the absence of publication bias among the studies.
Figure 13.
(a) Funnel plot on the incidence of mortality from six RCTs. (b & c) Funnel plots on the incidence of AEs and SAEs from two RCTs, respectively. (d & e) Funnel plots on the level of CRP and IL-6 from two RCTs, respectively.
Discussion
MSCs are well known for their regenerative, anti-inflammatory, and immunomodulatory properties that make it a promising cell-based treatment for many diseases. 27 Since past decades, MSCs derived from various tissue sources, such as BM, AT, and PT (such as umbilical cord), have been explored. 13 In view of the MSCs therapeutic properties, they have been tested in a great number of clinical research involving autoimmune disease, cancer, GvHD, and various other critical diseases.11,12 On top of the beneficial effects of MSC in inflammatory diseases, MSCs also have special advantages in the treatment for COVID-19. Considering the lung is the main organ targeted by SARS-CoV-2, the accumulation of MSCs in the lungs can facilitate the healing and regeneration of alveolar epithelial cells through various mechanisms when delivered systematically (e.g. intravenous infusion).28,29
In this systematic review and meta-analysis, the safety and efficacy of MSCs in treating severe COVID-19 was investigated. All MSCs used in the included studies were obtained from umbilical cord. Wharton’s Jelly-derived MSCs in particular were used in study by Adas et al. 21 Umbilical cord-derived mesenchymal stromal/stem cells (UC-MSCs) have a higher proliferation rate compared with other sources of MSC, such as BM and ATs. Therefore, the use of MSCs originating from umbilical cord is a practical approach for clinical studies. All the studies prepared the MSCs according to appropriate practice, guidelines, and regulations. The criteria of the MSCs, such as cell number, identity (immunophenotype), viability, safety (endotoxin, sterility, mycoplasma assays, microbiological tests, and cytogenetic analysis), and functionality (colony-forming potential and immunosuppressive effects) were also fulfilled in majority of the included studies.19–24 Shu et al. 25 and Zhu et al. 26 did not describe the preparation of MSCs in detail, but both studies used MSCs that had been approved by the Ethics Committee of Huangshi Hospital of Traditional Chinese Medicine and National Institutes for Food and Drug Control of China, respectively.
As for dose of MSCs, the number of cells given by each included study was not comparable due to different units of measurements (Table 3). Lanzoni et al. 19 and Liang et al. 23 reported the values in cells/infusion while the rest reported the values in cells/kg. The dose varies across the studies, but 1 × 106 cells/kg was the most common.20,22,26 Meanwhile, the doses in studies by Adas et al. 21 and Shu et al. 25 are 3 × 106cells/kg and 2 × 106 cells/kg, respectively. Furthermore, Lanzoni et al. 19 and Liang et al. 23 administered 100 ± 20 × 106 cells/infusion and 5 x 107cells/infusion, respectively. In a study by Rebelatto et al., 24 dose value of 5 × 105 cells/kg was administered. The study did not give any explanation on the low choice of dose. The effect of high, low, and multiple doses on the improvement of the outcomes could not be evaluated due to lack of data to enable proper comparison. Hence, the conclusion as to which choice of doses yield better results cannot be achieved.
Safety was evaluated based on the pooled effect estimate from meta-analysis on the incidence of mortality, AE, & SAEs. From a meta-analysis of six studies (Figure 8), it was observed that the risk of mortality is significantly lower (p = 0.03) in the MSC-treated group compared with the control group with overall RR of 0.65 (CI = 0.44, 0.96). This result corresponds to the mortality rate in Table 4, which demonstrated that four of six included RCTs showed lower mortality rate in MSC-treated compared with control groups.19,21,22,25 On the contrary, the remaining two RCTs showed higher mortality rate in the MSC-treated group than the control group, but with no significant difference.20,24 From these two studies, Rebelatto et al. 24 had a notable difference in the mortality incidence, which are five deaths from the MSC-treated group and one death from the control group. However, the study highlighted that there were baseline imbalances due to small sample size that showed the MSC-treated group was more compromised than the control group.
This might explain the higher number of deaths in the MSC-treated group. Some of the causes of deaths among the RCTs were secondary infection due to bacteria, bacterial septic shock, myocardial infarction, thromboembolism, acute respiratory failure, multi-organ dysfunction syndrome, and failed endotracheal intubation.19,21,24 It was also observed in a study by Dilogo et al. 22 that age and comorbidities led to early mortality among the patients. However, no treatment-related death was reported by any of the studies. In addition, the case reports showed no mortality as the patient from both reports recovered from COVID-19.23,26
With respect to incidence of AEs and SAEs, no statistical significance on the overall OR was observed between MSC-treated and control groups (Figures 9 and 10). Based on the figures, the incidence of AEs had an overall OR of 0.72 (CI = 0.07, 7.42) with p-value of 0.78. On the other hand, the incidence of SAEs had an overall odd ratio of 0.38 (CI = 0.03, 4.37) with p-value of 0.44. These results suggest that the MSC treatment had no association with the events. However, it must be highlighted that only two studies, Lanzoni et al. 19 and Monsel et al. 20 provided sufficient information that enabled inclusion in the meta-analysis. Even though this might question the accuracy of the results due to the small number of included studies, the total sample size was considered substantial to produce appreciable results. Nevertheless, it is undeniable that further reviews with more included studies need to be conducted to enhance the result accuracy. In these two studies, most of the AEs were deemed unrelated to the MSC treatment, but some of the reported possible infusion-associated AEs were diarrhea, increased vasopressor-dose, new cardiac arrhythmia, new ventricular tachycardia, ventricular fibrillation, or asystole.19,20 Among these AEs, only diarrhea and increased vasopressor dose due to worsening of existing bradycardia occurred in the MSC-treated group with one patient for each event. Other RCTs that were not included in the meta-analysis reported no serious or obvious complications after treatment except Rebelatto et al. 24 that reported mild AEs, but these AEs were deemed unrelated to the treatment. Both case reports by Liang et al. 23 and Zhu et al. 26 also reported no obvious adverse effects following MSC infusion. No treatment-related SAEs were reported from all studies.
Efficacy was observed based on the inflammatory response and pulmonary function among the COVID-19 patients. In evaluating the inflammatory response, levels of CRP and IL-6 were chosen as the analyzed outcomes in the meta-analysis considering that these cytokines are among the main pro-inflammatory cytokines in COVID-19 progression. 30 Only two studies were included in the meta-analysis for these outcomes, as the remaining studies did not provide sufficient information to enable analysis.21,24 Despite the small number of included studies, the total sample size was considered substantial to perform a meta-analysis. Based on Figures 11 and 12, both forest plots show no significant differences on the overall SMD of CRP [p = 0.06; SMD (CI) = –0.66 (–1.35, 0.03)] and IL-6 [p = 0.09; SMD (CI) = –0.58 (–1.27, 0.10)] between MSC-treated and control groups. Therefore, it cannot be deduced that MSCs are capable of reducing the inflammatory reaction in COVID-19 patients.
In spite of that, Table 5 shows that three of four RCTs that measured CRP reported lower levels of the protein in MSC-treated group compared with control group.21,24,25 Furthermore, both case reports by Liang et al. 23 and Zhu et al. 26 also reported decrease in the level of CRP after treatment. Levels of IL-6 were also observed to be lower in MSC-treated group in all five studies that measured the cytokine.19,21,22,24 In view of the results from Table 5, there is an indication that MSCs can reduce the levels of CRP and IL-6. Therefore, there are valid grounds to assume that statistical significance could be achieved if more studies were included in the meta-analysis.
Apart from CRP and IL-6, levels of other cytokines, such as PCT, TNF-α, IL-2, and IL-10 were also reported (Table 5). Lanzoni et al. 19 and Monsel et al. 20 observed significant decrease of TNF-α in MSC-treated group, but no significant differences were observed between both groups in studies by Adas et al. 21 and Rebelatto et al. 24 Decreasing trend was observed on the levels of PCT following MSC treatment in studies by Liang et al. 23 and Zhu et al. 26 Moreover, Adas et al. 21 reported that levels of PCT in the MSC-treated group were significantly lower than the control group. Contrarily, no significant difference between groups was observed in Dilogo et al. 22 As for IL-2, studies by Adas et al. 21 and Lanzoni et al. 19 reported decreasing trend on the level of the cytokine in MSC-treated group following treatment while no significant difference was observed between groups in a study by Rebelatto et al. 24
On the other hand, an increasing trend was observed for anti-inflammatory cytokine, IL-10 in the MSC-treated group.21,22 In the study by Dilogo et al., 22 the increasing trend of IL-10 was observed for up to 7 days following MSC infusion. Furthermore, Rebelatto et al. 24 reported a higher level of IL-10 in the MSC-treated group compared with the control group throughout a period of 4 months. Conversely, Monsel et al. 20 reported a decreasing trend in IL-10, but the decline was observed later (D7 and D14) than the decline of pro-inflammatory cytokines in the control group (D4 and D7). To sum up, a decreasing pattern can be observed for pro-inflammatory cytokines (i.e. CRP, PCT, IL-6, TNF-α, and IL-2) and an increasing pattern can be observed for anti-inflammatory cytokine, IL-10. Another factor that might influence the results includes the effect of dexamethasone as a potential confounding variable that can reduce the inflammatory reaction in the patient that has been addressed by Adas et al. 21 Other studies also administered concomitant treatments, such as corticosteroids, convalescent plasma, and glucocorticoids, which might help in reducing the inflammatory reaction in the patients. All in all, more studies are needed to evaluate the effect of MSCs on the level of these inflammatory cytokines and markers.
In evaluating pulmonary function, oxygenation status and CT features from all applicable studies were extracted and observed (Table 6). Unfortunately, meta-analysis was not performed due to lack of data. Oxygenation status was mainly observed based on the PaO2/FiO2-ratio, which was found to be lower in MSC-treated group compared with the control group with no significant differences.20,21 The lack of differences may be due to the severe respiratory conditions among the patients. If the conditions were severe to the point where ventilator supports were required, the influence of MSCs in improving the ratio might be reduced due to lung damage. However, Monsel et al. 20 also reported that there was significant increase in PaO2/FiO2-ratio from D0 to D7 only in the MSC-treated group, indicating that MSCs can improve oxygenation patterns in critically ill COVID-19 patients. Nonetheless, it must be noted that baseline imbalances that might favor the MSC-treated group were observed in the study.
Furthermore, Adas et al. 21 reported that the duration of ventilator usage in the MSC-treated group was lower than the control group with the presence of statistical significance. In line with this statement, Shu et al. 25 found that a higher percentage of patients in the MSC-treated group were independent of supplemental oxygen when compared with the control group. Apart from that, the study also found that the time taken for oxygenation index to return to normal in the MSC-treated group was significantly shorter than the control group. Furthermore, in the case report by Zhu et al., 26 the SpO2 of the patient returned to normal on the second day following MSC infusion. With regard to CT features, Table 6 shows that all studies that conducted CT scans on the patients demonstrated improvement in the lungs following MSC infusion. This was indicated by the reduction in GGO, consolidation, pneumonia infiltration, and the number of lobes involved as well as improvement in CT scores.23–26 In brief, MSCs were found to be able to improve lung clearance based on the CT scan.
It is noteworthy to mention here that although we have found preliminary signs of safety and efficacy in eight published clinical studies, some of the potential risks of MSC transplant have been reported in the treatment of chronic GvHD, hemorrhagic cystitis, and ARDS. In a study by Ringdén et al., 31 the application of MSCs appeared to be safe in treating acute GvHD, hemorrhages and ARDS. However, their procoagulant properties have not been anticipated for clinical use. In case of COVID-19, MSC-derived products have shown to express variable concentrations of highly procoagulant tissue factor (TF/CD142), which compromise the hemocompatibility and safety profile of the cells, which triggers blood clotting and further promote the risk of thromboembolism. 32 In that view, well-characterized MSC-based products with potent manufacturing techniques and improved clinical delivery modes hold significant potential for ameliorating COVID-19. Despite the evidential need of MSC therapy in COVID-19, it is crucial to integrate the compatibility testing of both innate and adaptive immune system into the current guidelines of MSC transplantation for developing safe and efficacious therapies. 33
Limitation of current systematic review and meta-analysis
The main limitation of this review is the limited number of completed trials related to the topic of interest. Most of the clinical trials are still ongoing. Therefore, the data that can be used for meta-analysis were insufficient. As a result, this review had failed to analyze the data for pulmonary function. Furthermore, the lack of trials also resulted in small total sample size in the meta-analysis for a few outcomes, such as the levels of CRP and IL-6, as well as the incidence of AEs and SAEs. Even though the total sample size was considered substantial to perform meta-analysis, it is preferable to include more trials for a larger total sample size. Besides the sample size and number of trials, there are possibilities of substantial bias arising from the screening process as 70 articles were removed due to failure in full-text retrieval. This lack of access to potentially relevant articles could affect the outcome of this study. On top of that, two studies also reported baseline imbalances that might favor either MSC-treated group or control group due to small sample size.19,20,24 This could also have exerted certain bias on the study results. Apart from that, the administration of additional treatments, such as corticosteroids (e.g. dexamethasone and hydrocortisone) and convalescent plasma should also be considered as potential confounding variables in evaluating the outcomes. However, this review did not investigate the effect of simultaneous use of both MSCs and other treatments. Other factors that need to be taken into account are preparation, handling and transport time of MSC following release of cells from processing facilities until their administration into patients. Prolonged transport and storage time may reduce the cell viability and treatment efficacy. These factors need to be considered as low cell viability may cause complications to the patients, such as inflammation due to dead cell debris. 34 This could be an important consideration for further study.
Conclusion
The MSC-treated group was associated with lower risk of mortality compared with the control group but was unrelated to the incidence of AEs and SAEs. In addition, descriptive analysis indicates that MSCs have the potential to alleviate inflammatory responses and improve lung clearance and oxygenation in COVID-19 patients. These imply that MSCs are a promising and safe therapeutic strategy for COVID-19 treatment. However, this still remain inconclusive due to insufficient number of studies resulting in the lack of data for analysis. In view of this limitation, systematic review and meta-analysis with more included studies and data retrievability need to be conducted in the near future.
Supplemental Material
Supplemental material, sj-docx-1-tar-10.1177_17534666231158276 for Systematic review and meta-analysis of mesenchymal stromal/stem cells as strategical means for the treatment of COVID-19 by Hannah Taufiq, Kamal Shaik Fakiruddin, Umaiya Muzaffar, Moon Nian Lim, Syahnaz Rusli, Nor Rizan Kamaluddin, Ezalia Esa and Syahril Abdullah in Therapeutic Advances in Respiratory Disease
Acknowledgments
The authors wish to thank the Director General of Health, Malaysia for his permission to publish this paper.
Footnotes
ORCID iD: Umaiya Muzaffar  https://orcid.org/0000-0002-6785-3388
https://orcid.org/0000-0002-6785-3388
Data sharing statement: The data are available from the corresponding authors upon reasonable request.
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Hannah Taufiq, Department of Biomedical Sciences, Faculty of Medicine and Health Sciences, Universiti Putra Malaysia, Serdang, Selangor, Malaysia.
Kamal Shaik Fakiruddin, Haematology Unit, Cancer Research Centre, Institute for Medical Research (IMR), National Institutes of Health (NIH), Ministry of Health Malaysia, Shah Alam, Selangor, Malaysia.
Umaiya Muzaffar, UPM-MAKNA Cancer Research Laboratory, Institute of Bioscience, Universiti Putra Malaysia, Serdang, Selangor, Malaysia.
Moon Nian Lim, Haematology Unit, Cancer Research Centre, Institute for Medical Research (IMR), National Institutes of Health (NIH), Ministry of Health Malaysia, Shah Alam, Selangor, Malaysia.
Syahnaz Rusli, Department of Biomedical Sciences, Faculty of Medicine and Health Sciences, Universiti Putra Malaysia, Serdang, Selangor, Malaysia.
Nor Rizan Kamaluddin, Haematology Unit, Cancer Research Centre, Institute for Medical Research (IMR), National Institutes of Health (NIH), Ministry of Health Malaysia, Shah Alam, Selangor, Malaysia.
Ezalia Esa, Haematology Unit, Cancer Research Centre, Institute for Medical Research (IMR), National Institutes of Health (NIH), Ministry of Health Malaysia, Shah Alam, Selangor, Malaysia.
Syahril Abdullah, Department of Biomedical Sciences, Faculty of Medicine and Health Sciences, Universiti Putra Malaysia, Serdang 43400, Selangor, Malaysia; UPM-MAKNA Cancer Research Laboratory, Institute of Bioscience, Universiti Putra Malaysia, Serdang 43400, Selangor, Malaysia.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contributions: Hannah Taufiq: Conceptualization; Data curation; Methodology; Software; Validation.
Kamal Shaik Fakiruddin: Data curation; Formal analysis; Funding acquisition; Investigation; Project administration; Supervision; Validation; Visualization; Writing – review & editing.
Umaiya Muzaffar: Investigation; Visualization; Writing – original draft; Writing – review & editing.
Moon Nian Lim: Writing – review & editing.
Syahnaz Rusli: Conceptualization; Data curation; Methodology; Software; Validation.
Nor Rizan Kamaluddin: Writing – review & editing.
Ezalia Esa: Writing – review & editing.
Syahril Abdullah: Data curation; Formal analysis; Funding acquisition; Investigation; Project administration; Supervision; Validation; Visualization; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by the Ministry of Health (MOH) of Malaysia, grant number NMRR ID-22-00166-R82
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Availability of data and materials: Not applicable.
References
- 1.Cucinotta D, Vanelli M.WHO declares COVID-19 a pandemic. Acta Biomedica 2020; 91: 157–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 2020; 395: 565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nguyen HL, Lan PD, Thai NQ, et al. Does SARS-CoV-2 bind to human ACE2 more strongly than does SARS-CoV? J Phys Chem B 2020; 124: 7336–7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shang J, Ye G, Shi K, et al. Structural basis of receptor recognition by SARS-CoV-2. Nature 2020; 581: 221–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng J.SARS-coV-2: an emerging coronavirus that causes a global threat. Int J Biol Sci 2020; 16: 1678–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.SARS-CoV-2 variant classifications and definitions, https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-classifications.html
- 7.Dennis JE, Carbillet JP, Caplan AI, et al. The STRO-1+ marrow cell population is multipotential, 2002, www.karger.com [DOI] [PubMed]
- 8.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006; 8: 315–317. [DOI] [PubMed] [Google Scholar]
- 9.Kim EJ, Kim N, Cho SG.The potential use of mesenchymal stem cells in hematopoietic stem cell transplantation. Exp Mol Med 2013; 45: e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sarugaser R, Hanoun L, Keating A, et al. Human mesenchymal stem cells self-renew and differentiate according to a deterministic hierarchy. PLoS ONE 2009; 4: e6498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim N, Cho SG.Clinical applications of mesenchymal stem cells. Korean J Intern Med 2013; 28: 387–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levy O, Kuai R, Siren EMJ, et al. Shattering barriers toward clinically meaningful MSC therapies. Science Advances 2020; 6: 1–18. https://www.science.org/doi/10.1126/sciadv.aba6884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moll G, Ankrum JA, Kamhieh-Milz J, et al. Intravascular mesenchymal stromal/stem cell therapy product diversification: time for new clinical guidelines. Trends Mol Med 2019; 25: 149–163. [DOI] [PubMed] [Google Scholar]
- 14.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372: n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amir-Behghadami M, Janati A.Population, Intervention, Comparison, Outcomes and Study (PICOS) design as a framework to formulate eligibility criteria in systematic reviews. Emerg Med J 2020; 37: 387–387. [DOI] [PubMed] [Google Scholar]
- 16.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019; 366: l4898. [DOI] [PubMed] [Google Scholar]
- 17.McGuinness LA, Higgins JPT. Risk-of-bias VISualization (robvis): an R package and Shiny web app for visualizing risk-of-bias assessments. Res Synth Methods 2021; 12: 55–61. [DOI] [PubMed] [Google Scholar]
- 18.Checklist for case reports, 2017, https://jbi.global/critical-appraisal-tools
- 19.Lanzoni G, Linetsky E, Correa D, et al. Umbilical cord mesenchymal stem cells for COVID-19 acute respiratory distress syndrome: a double-blind, phase 1/2a, randomized controlled trial. Stem Cells Transl Med 2021; 10: 660–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Monsel A, Hauw-Berlemont C, Mebarki M, et al. Treatment of COVID-19-associated ARDS with mesenchymal stromal cells: a multicenter randomized double-blind trial. Crit Care 2022; 26: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adas G, Cukurova Z, Yasar KK, et al. The systematic effect of mesenchymal stem cell therapy in critical COVID-19 patients: a prospective double controlled trial. Cell Transplant 2021; 30:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dilogo IH, Aditianingsih D, Sugiarto A, et al. Umbilical cord mesenchymal stromal cells as critical COVID-19 adjuvant therapy: a randomized controlled trial. Stem Cells Transl Med 2021; 10: 1279–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liang B, Chen J, Li T, et al. Clinical remission of a critically ill COVID-19 patient treated by human umbilical cord mesenchymal stem cells a case report. Medicine 2020; 99: 31. http://chinaxiv.org/abs/202002.00084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rebelatto CLK, Senegaglia AC, Franck CL, et al. Safety and long-term improvement of mesenchymal stromal cell infusion in critically COVID-19 patients: a randomized clinical trial. Stem Cell Res Ther 2022; 13: 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shu L, Niu C, Li R, et al. Treatment of severe COVID-19 with human umbilical cord mesenchymal stem cells. Stem Cell Res Ther 2020; 11: 361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu Y, Zhu R, Liu K, et al. Human umbilical cord mesenchymal stem cells for adjuvant treatment of a critically ill COVID-19 patient: a case report. Infect Drug Resist 2020; 13: 3295–3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fan XL, Zhang Y, Li X, et al. Mechanisms underlying the protective effects of mesenchymal stem cell-based therapy. Cell Mol Life Sci 2020; 77: 2771–2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee RH, Pulin AA, Seo MJ, et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell 2009; 5: 54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Masterson CH, Curley GF, Laffey JG.Modulating the distribution and fate of exogenously delivered MSCs to enhance therapeutic potential: knowns and unknowns. Intensive Care Med Exp 2019; 7: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lavillegrand JR, Garnier M, Spaeth A, et al. Elevated plasma IL-6 and CRP levels are associated with adverse clinical outcomes and death in critically ill SARS-CoV-2 patients: inflammatory response of SARS-CoV-2 patients. Ann Intensive Care 2021; 11: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ringdén O, Moll G, Gustafsson B, et al. Mesenchymal stromal cells for enhancing hematopoietic engraftment and treatment of graft-versus-host disease, hemorrhages and acute respiratory distress syndrome. Front Immunol 2022; 13: 839844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moll G, Drzeniek N, Kamhieh-Milz J, et al. MSC therapies for COVID-19: importance of patient coagulopathy, thromboprophylaxis, cell product quality and mode of delivery for treatment safety and efficacy. Front Immunol 2020; 11: 1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moll G, Ankrum JA, Olson SD, et al. Improved MSC minimal criteria to maximize patient safety: a call to embrace tissue factor and hemocompatibility assessment of MSC products. Stem Cells Transl Med 2022; 11: 2–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garvican ER, Cree S, Bull L, et al. Viability of equine mesenchymal stem cells during transport and implantation. Stem Cell Res Ther 2014; 5: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tar-10.1177_17534666231158276 for Systematic review and meta-analysis of mesenchymal stromal/stem cells as strategical means for the treatment of COVID-19 by Hannah Taufiq, Kamal Shaik Fakiruddin, Umaiya Muzaffar, Moon Nian Lim, Syahnaz Rusli, Nor Rizan Kamaluddin, Ezalia Esa and Syahril Abdullah in Therapeutic Advances in Respiratory Disease