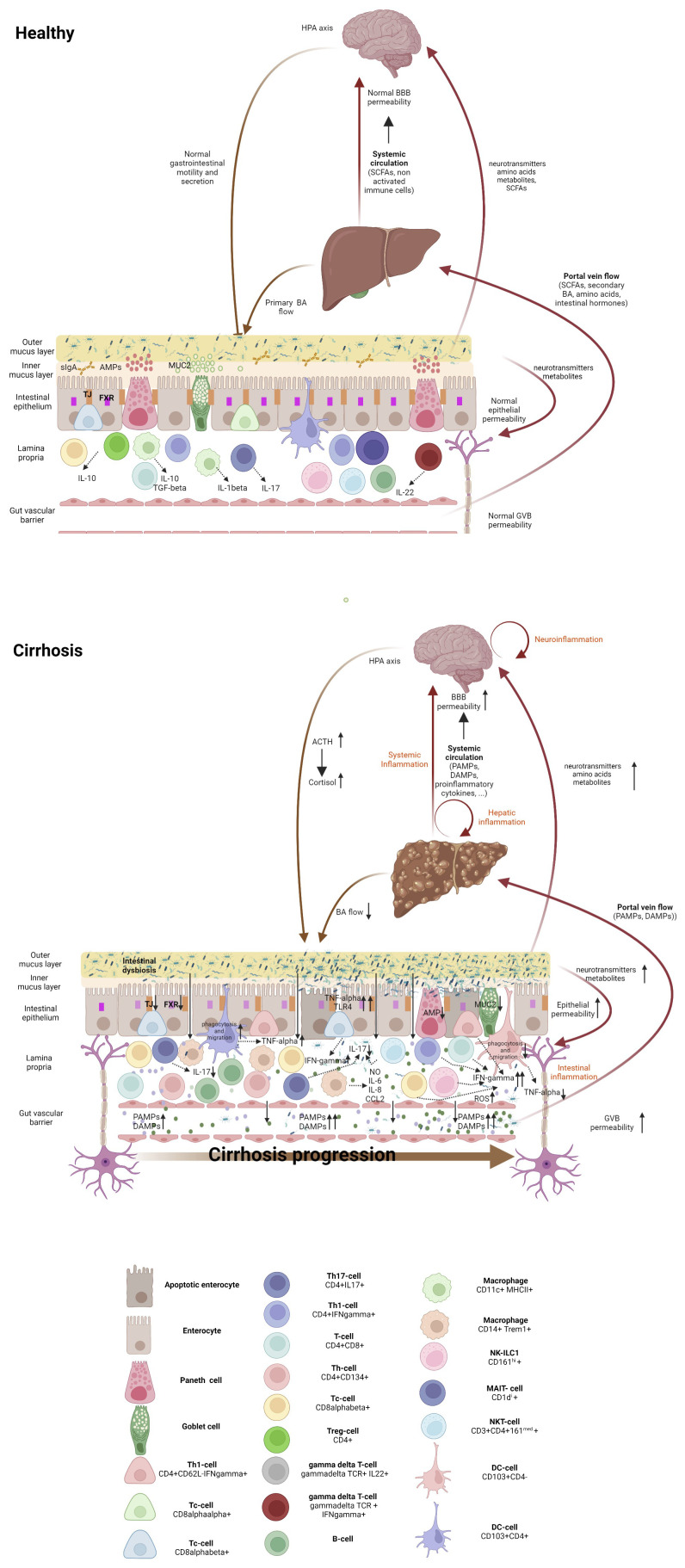
Figure 1.
Gut-liver-brain axis in health and cirrhosis, highlighting the intestinal immune milieu. The intestinal epithelial barrier is a multi-layered structure that separates bacteria in the intestinal lumen from the systemic circulation. In healthy individuals, eubiosis, a tolerant immune system and an efficient innate antibacterial defense maintain the integrity of the epithelial and vascular barriers. Cirrhosis is associated with an altered microbiome which results from decreased bile flow, with deficient levels of primary but increased of secondary bile acids, together with intestinal hypomotility. Gut dysbiosis drives bacterial colonization of the inner mucous layer of the intestine, which facilitates the interaction of bacteria with the immune system, resulting in intestinal inflammation with recruitment and activation of immune system cells. Composition and severity of intestinal inflammation change with cirrhosis progression. Intestinal inflammation in the compensated stage is featured by activated innate cells, including DCs producing TNF-α and showing increased phagocytic and migratory abilities, along with the activation of the adaptive immune system, mainly located in the intraepithelial compartment. In the decompensated stage, intestinal immune system derangement is characterized by non-activated DCs, with lowered TNF-α secretion, and deficient phagocytosis and migration abilities, as well as expansion of activated macrophages and Th1 lymphocytes with concomitant Th17 reduction. Inflammation results in the release of proinflammatory cytokines and ROS, which worsens epithelial and the vascular barrier damage, hyperpermeability and access of bacterial products to the liver (via portal vein Flow) and to the systemic circulation. Elevated systemic levels of proinflammatory cytokines, PAMPs and DAMPs accelerate cirrhosis progression and increase the blood-brain-barrier permeability, inducing neuroinflammation (astrocyte swelling, activated microglia and immune system infiltration), which activates hypothalamic-pituitary-adrenal axis, and affects intestinal barrier integrity. Within the gut, the altered microbiota can produce neurotransmitters, amino acids and microbial metabolites. These metabolites can travel through portal circulation to interact with the host immune system, influence metabolism and/or affect local neuronal cells of the enteric nervous system and afferent pathways of the vagus nerve that signal directly to the brain. DC, dendritic cell; Treg, regulatory T cells; Tc, cytotoxic T cell; Th, helper T cell; ILC, innate lymphoid cell; NK cell, natural killer cell; NKT cell, natural killer T cell, MAIT cell, mucosal-associated invariant T cell, AMP, antimicrobial peptides; MUC-2, mucin-2; FXR, Farnesoid X receptor, TJ, Tight junction, BA, bile acid; NO, nitric oxide; IL-, interleukin; CCL2, C-C motif ligand 2; PAMPs, pathogen-associated molecular patterns; 318 DAMPs, damage-associated molecular patterns, ROS, radical oxygen species; TNF-γ, tumor necrosis factor-alpha; IFN-γ, interferon γ; ACTH, adrenocorticotrophic hormone; BBB, blood-brain barrier; HPA, hypothalamic-pituitary-adrenal.