Table 2.
LS-Sc3+ promoted the Michael addition/dehydration tandem reaction between 3-acetyl-2-hydroxy-2-methylchromenes and indoles [33] 1.
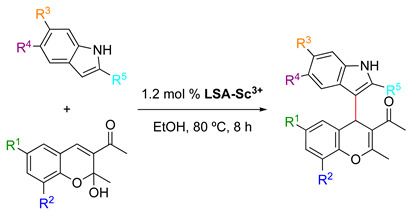
| |||||||
|---|---|---|---|---|---|---|---|
| Entry | Catalyst | R1 | R2 | R3 | R4 | R5 | Yield (%) 2 |
| 1 | LSA-Sc3+ | Br | H | H | H | H | 93 (90) |
| 2 | LSA-Sc3+ | Br | H | H | H | Me | 98 |
| 3 | LSA-Sc3+ | Br | H | H | OMe | H | 97 |
| 4 | LSA-Sc3+ | Br | H | H | Br | H | 61 |
| 5 | LSA-Sc3+ | Br | H | Me | H | H | 93 |
| 6 | LSA-Sc3+ | Br | H | H | H | Ph | 85 3 |
| 7 | LSA-Sc3+ | Cl | H | H | H | H | 96 |
| 8 | LSA-Sc3+ | Cl | H | H | H | Me | 96 |
| 9 | LSA-Sc3+ | H | H | H | H | H | 93 |
| 10 | LSA-Sc3+ | OMe | H | H | H | H | 86 |
| 11 | LSA-Sc3+ | tBu | H | H | H | H | 96 |
| 12 | LSA-Sc3+ | H | Me | H | H | H | 68 |
| 13 | LSA-Sc3+ | H | OMe | H | H | H | 61 |
| 14 | LSA-Sc3+ | Me | Me | H | H | H | 83 |
| 15 | SiO2-Sc3+ | Br | H | H | H | H | 92 (50) |
| 16 | resin-Sc3+ | Br | H | H | H | H | 87 (63) |
1 Reaction conditions: 3-acetyl-2-hydroxy-2-methylchromene derivative (0.5 mmol), indole derivative (0.6 mmol), LSA-Sc3+ (1.2 mol%), EtOH (1 mL), 80 °C, 8 h. Isolated yields. The yield in parentheses shows the reuse of the catalyst in the third run. 2 Isolated yield. 3 Catalyst amount = 2 mol%. 1-Methyl-2-phenyl-1H-indole was used as the Michael donor.
