Table 3.
LSA-Sc3+ catalyzed electrophilic ring-opening reactions of 2-butoxy-3,4-dihydropyrans with indoles [33] 1.
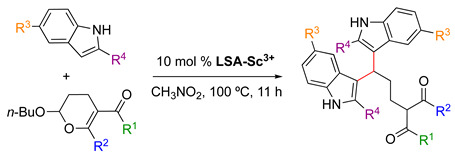
| |||||
|---|---|---|---|---|---|
| Entry | R1 | R2 | R3 | R4 | Yield (%) 2 |
| 1 | OEt | Me | H | H | 89 (88) |
| 2 | OEt | Me | Br | H | 86 |
| 3 | OEt | Ph | H | H | 89 |
| 4 | OEt | Ph | Br | H | 63 |
| 5 | OMe | Me | H | H | 87 |
| 6 | OMe | Me | H | Me | 80 |
| 7 | OMe | Me | Br | H | 90 |
| 8 | Me | Me | H | H | 95 |
| 9 | Me | Me | Br | H | 96 |
| 10 | OCH2CH2OMe | Me | H | H | 87 |
1 Reaction conditions: 2-butoxy-3,4-dihydropyran derivative (0.5 mmol), indole derivative (1.25 mmol), LSA-Sc3+ (10 mol%), CH3CN2 (1 mL), 100 °C, 11 h. Isolated yields. The yield in parentheses shows reuse of the catalyst in the third run. 2 Isolated yield.
