Table 4.
LSA-FAS-Cu2+ catalyzed three-component reactions of 4-aminoindoles, alkynes, and aldehydes [34] 1.
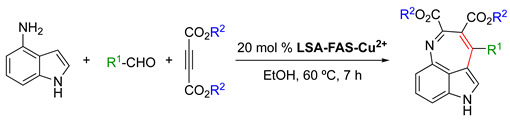
| |||
|---|---|---|---|
| Entry | R1 | R2 | Yield (%) 2 |
| 1 | 4-Me-Ph | Et | 86 |
| 2 | Ph | Et | 61 |
| 3 | 4-Br-Ph | Et | 46 |
| 4 | 4-CF3-Ph | Et | 64 |
| 5 | 2-OMe-Ph | Et | 55 |
| 6 | 2-NaPh | Et | 60 |
| 7 | 2-Cl,3-Cl-Ph | Et | 42 |
| 8 | (3-OMe,4-OMe,5-OMe)-Ph | Et | 37 |
| 9 |
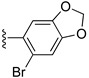
|
Et | 64 |
| 10 | CH2CH2-Ph | Et | 45 |
| 11 | Me | Et | 80 |
| 12 | Pr | Et | 74 |
| 13 | CH2tBu | Et | 71 |
| 14 |
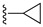
|
Et | 77 |
| 15 | 4-Me-Ph | Me | 65 |
1 Reaction conditions: 4-aminoindole/aldehyde/alkyne = 1.5:1:1.5, LSA-FAS-Cu2+ (20 mol%), EtOH (1 mL), 60 °C, 7 h. 2 Isolated yield.
