Table 5.
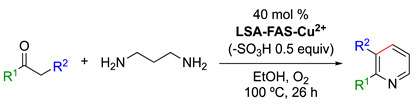
LSA-FAS-Cu2+ catalyzed the reaction of acetophenones and 1,3-diaminopropane, affording 2-arylpyridine derivatives [34] 1.
| |||
|---|---|---|---|
| Entry | R1 | R2 | Yield (%) 2 |
| 1 | Ph | H | 74 |
| 2 | 4-Me-Ph | H | 71 |
| 3 | 4-n-Pr-Ph | H | 70 |
| 4 | 4-n-amyl-Ph | H | 68 |
| 5 | 4-t-Bu-Ph | H | 63 |
| 6 | 4-Cy-Ph | H | 60 |
| 7 | 4-SMe-Ph | H | 82 |
| 8 | 4-OMe-Ph | H | 65 |
| 9 | 4-OBn-Ph | H | 67 |
| 10 | 4-Ph-Ph | H | 63 |
| 11 | 4-F-Ph | H | 45 |
| 12 | 4-Cl-Ph | H | 40 |
| 13 | 4-I-Ph | H | 39 |
| 14 | 3-Me-Ph | H | 65 |
| 15 | 2-NaPh | H | 58 |
| 16 | (3-Me,5-Me)-Ph | H | 68 |
| 17 | (3-OMe,4-OMe)-Ph | H | 56 |
| 18 | (3-OMe,4-OMe,5-OMe)-Ph | H | 73 |
| 19 |
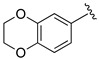
|
H | 64 |
| 20 |
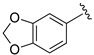
|
H | 71 |
| 21 |
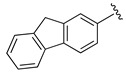
|
H | 69 |
| 22 |
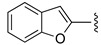
|
H | 76 |
| 23 |
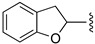
|
H | 75 |
1 Reaction conditions: ketone (0.2 mmol), 1-3-diaminopropane (0.6 mmol), acidified LSA-FAS-Cu2+ [(40 mol%), −SO3H (0.5 equiv)], O2 (1 atm), EtOH (1 mL), 100 °C, 26 h. Isolated yields. Note: For the sake of clarity, the group R2 is indicated in the reaction scheme, albeit R2 = H for all the examples. 2 Isolated yield.
