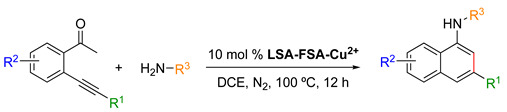
Table 6.
LSA-FAS-Cu2+-catalyzed synthesis of aminonaphthalene derivatives [34] 1.
| ||||
|---|---|---|---|---|
| Entry | R1 | R2 | R3 | Yield (%) 2 |
| 1 | Ph | H | 4-Me-Ph | 81 |
| 2 | Ph | H | 4-OMe-Ph | 85 |
| 3 | Ph | H | 4-t-Bu-Ph | 79 |
| 4 | Ph | H | 4-Br-Ph | 55 |
| 5 | Ph | H | (3-OMe,4-OMe)-Ph | 66 |
| 6 | Ph | H | 1-naphthalene | 70 |
| 7 | Ph | H | Bn | 61 |
| 8 | Ph | H | n-Bu | 83 |
| 9 | Ph | H |
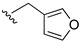
|
88 |
| 10 | Ph | H |
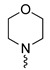
|
81 |
| 11 | Ph | Cl | 4-Me-Ph | 78 |
| 12 | Ph | F | 4-Me-Ph | 63 |
| 13 | 4-F-Ph | H | 4-Me-Ph | 71 |
| 14 | n-hexyl | H | 4-Me-Ph | 54 |
| 15 | cyclohexenyl | H | 4-MePh | 42 |
| 16 | Ph | H | 4-NO2Ph | n.d. |
| 17 | 4-OMe-Ph | H | 4-Me-Ph | n.d. |
1 Reaction conditions: ketone (0.2 mmol), amine (0.24 mmol), LSA-FAS-Cu2+ (10 mol%), DCE (1 mL), 100 °C, 12 h. 2 Isolated yield.
