Table 7.
LSA-PdNPs catalyzed the Suzuki–Miyaura cross-coupling reaction between aryl halides and phenyl boronic acids [71] 1.
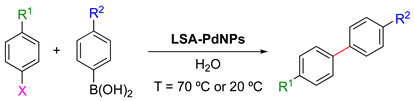
| |||||||
|---|---|---|---|---|---|---|---|
| Entry | X | R1 | R2 | Time (h) | T (°C) | Aryl Halide Conv. (%) 2 | Yield (%) 2 |
| 1 | I | H | H | 5 | 70 | 100 | 100 |
| 2 | I | H | H | 12 | 20 | 100 | 100 |
| 3 | CH=CH2 | H | H | 0.5 | 70 | 100 | 100 |
| 4 | CH=CH2 | H | H | 2 | 20 | 100 | 100 |
| 5 | I | NH2 | H | 12 | 70 | 70 | 28 |
| 6 | I | NH2 | H | 12 | 20 | 0 | - |
| 7 | Br | H | H | 12 | 70 | 100 | 80 |
| 8 | Br | H | H | 12 | 20 | 15 | 10 |
| 9 | Br | OMe | H | 12 | 70 | 90 | 20 |
| 10 | Br | OMe | H | 12 | 20 | 90 | 25 |
| 11 | I | H | CH2OH | 12 | 70 | 100 | 100 |
| 12 | I | H | CH2OH | 12 | 20 | 84 | 84 |
| 13 | Br | H | CH2OH | 12 | 70 | 95 | 75 |
| 14 | Br | H | CH2OH | 12 | 20 | 20 | 15 |
| 15 | Cl | H | CH2OH | 12 | 70 | 10 | 5 |
| 16 | Cl | H | CH2OH | 12 | 20 | 18 | 12 |
1 Reaction conditions: aryl halide (0.5 mmol), phenyl boronic acid derivative (0.5 mmol), K2CO3 (1.75 mmol), H2O (5 mL), aryl halide: LSA-PdNPs = 450:1 (molar ratio). Reaction times and temperatures as indicated. 2 Calculated by GC-MS.
