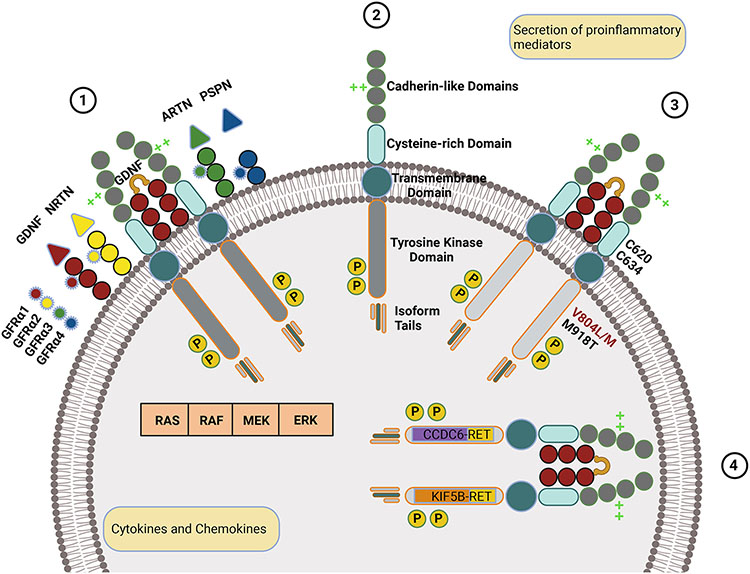
Fig. 1.
RET mechanisms in normal and oncogenic states. (1) Wild-type RET is a transmembrane protein with extracellular cadherin-like and cysteine-rich domains and an intracellular tyrosine kinase domain and isoform-specific tail. (2) Upon binding of the wild-type RET to a GFRα bound to a GDNF family ligand, RET homodimerizes, is autophosphorylated, and activates signaling through RAS, RAF, MEK, or ERK pathways. Ligands are color-coded (GDNF in red, NRTN in yellow, ARTN in green, and PSPN in blue), and respective GFRα receptors are color-matched. Calcium-binding is represented by bright green crosses and phosphorylation by the letter p in a yellow circle. (3) RET with oncogenic mutations, such as those indicated, is constitutively active. (4) Oncogenic RET with two of the most common fusions, CCDC6 and KIF58, is intracellular, undergoes ligand-independent dimerization, and is constitutively active.