Abstract
BACKGROUND:
In adults, vestibular loss is associated with cognitive deficits; however, similar relationships have not been studied in children.
OBJECTIVE:
Evaluate the effect of vestibular loss on working memory and executive function in children with a cochlear implant (CCI) compared to children with normal hearing (CNH).
METHODS:
Vestibular evoked myogenic potential, video head impulse, rotary chair, and balance testing; and the following clinical measures: vision, hearing, speech perception, language, executive function, and working memory.
RESULTS:
Thirty-eight CNH and 37 CCI participated (26 with normal vestibular function, 5 with unilateral vestibular loss, 6 with bilateral vestibular loss). Children with vestibular loss demonstrated the poorest balance performance. There was no significant reduction in working memory or executive function performance for either CCI group with vestibular loss; however, multivariate regression analysis suggested balance performance was a significant predictor for several working memory subtests and video head impulse gain was a significant predictor for one executive function outcome.
CONCLUSIONS:
CCI with vestibular loss did not have significantly reduced working memory or executive function; however, balance performance was a significant predictor for several working memory subtests. Degree of hearing loss should be considered, and larger sample sizes are needed.
Keywords: vestibular, cognition, working memory
INTRODUCTION:
In children, vestibular loss is associated with both sensorineural hearing loss (O’Reilly et al., 2010; Li et al., 2016) and gross motor delay (Inoue et al., 2013; Janky et al., 2018a; Rine et al., 2000; Rine et al., 2004; Janky & Givens, 2015; Kimura et al., 2017). Vestibular loss is estimated to occur in 50% of children with severe-to-profound sensorineural hearing loss (Cushing et al., 2013; Janky & Givens, 2015; Janky et al., 2018a). Of those, approximately 25% are estimated to have bilateral vestibular loss (Cushing et al., 2013). Children with vestibular loss have delays in meeting their gross motor milestones, and if not treated with physical therapy, these delays can be progressive in nature (Rine et al., 2000).
In addition to gross motor delay, several investigations have noted a relationship between vestibular loss and reading/academic outcomes in children with hearing loss and those without hearing loss. Children with vestibular loss have difficulty seeing clearly while their head is in motion, as demonstrated by reduced dynamic visual acuity (Braswell & Rine, 2006; Martin et al., 2012; Rine et al., 2003; Rine et al., 2013; Janky & Givens, 2015), which has been linked to poor reading acuity (Braswell & Rine, 2006). Additionally, vestibular loss has been associated with other academic deficits related to learning and reading. Regardless of hearing status, vestibular abnormalities have been noted in 40% of children with reading difficulties compared to 10% of normal control children (Snashall, 1983). A higher rate of vestibular involvement and postural sway has also been documented in children with learning difficulties as well as children underperforming in school (Franco et al., 2007; Franco et al., 2008; Tomaz et al., 2014).
In adults, vestibular loss has been associated with cognitive deficits. Specifically, vestibular loss in adults is associated with decreased spatial navigation (Xie et al., 2017), spatial memory (Kremmyda et al., 2016), visuospatial ability (Guidetti et al., 2020; Smith et al., 2019; Popp et al., 2017; Ayar et al., 2020), attention (Bigelow et al., 2015; Dobbels et al., 2019), executive function (Popp et al., 2017), and processing speed (Popp et al., 2017), among others. While children with vestibular loss exhibit many of the same consequences as adults with vestibular loss (i.e., delays in gross motor development, increased fall risk, reduced dynamic visual acuity; Braswell & Rine, 2006; Inoue et al., 2013; Janky et al., 2018a; Janky & Givens, 2015; Kimura et al., 2017; Martin et al., 2012; Rine et al., 2000; Rine et al., 2003; Rine et al., 2004; Rine et al., 2013), the relationship between cognition and vestibular loss in children has not been widely studied. Lacroix et al. (2020) noted reduced performance on measures of visuospatial working memory, mental rotation and space orientation, but not visual attention; however, their children represented a small heterogeneous cohort (n = 13), half with hearing loss necessitating either a hearing aid or a cochlear implant and the remaining children with normal hearing.
While working memory and executive function deficits have been reported in children with cochlear implants (Beer et al., Nittrouer et al., 2013; Nittrouer et al., 2017), the contribution of vestibular function to the cognitive abilities of this group of children has not yet been described. Vestibular loss that affects a child’s working memory and executive function abilities could lead to poor academic performance. Therefore, the purpose of this study was to quantify the degree of vestibular loss in school-age children with a cochlear implant (CCI) and evaluate the effect of vestibular loss on working memory and executive function compared to children with normal hearing (CNH). We hypothesized that like adults with vestibular loss, vestibular loss in CCI would result in visuospatial working memory and executive function deficits not explained by hearing loss alone.
METHODS:
Participants:
To qualify for the current study, children were required to have nonverbal intelligence scores within the range of 1.5 SD below the mean or better to ensure non-verbal intelligence was not a factor driving group differences in the cognitive outcomes measured, namely working memory and executive function. Nonverbal intelligence was assessed with the Wechsler Abbreviated Scale of Intelligence-II (WASI-II; Wechsler & Zhou, 2011) using the block design and matrix reasoning subtests, both of which tap an individual’s fluid intelligence. In the block design subtest, two-color blocks are used to recreate a two-dimensional pattern within a specified amount of time. In the matrix reasoning subtest, participants select the picture item that completes a matrix. Scores from the block design and matrix reasoning subtests comprise the Perceptual Reasoning Index (PRI) composite score, which had to be ≥ 77 for inclusion. Three CCI were excluded due to low PRI composite scores.
In total, 38 CNH (mean age: 12.2 years, range 7 – 18) and 37 CCI (mean age: 13.4, range 7 – 20) participated in the study. By case history, all CNH denied hearing loss or history of dizziness, imbalance or other neurologic complaints. Of the CCI, 25 were bilaterally implanted and 12 were unilaterally implanted. Of the 12 children implanted unilaterally, 6 used a hearing aid and 6 used no form of amplification on the contralateral side. Average age of implantation was 38 months (range 10 – 156 months). Two children had delayed implantation (156 months) due to progressive hearing loss. Hearing loss etiology in the CCI was unknown in 20, malformation (i.e., enlarged vestibular aqueduct, Pendred Syndrome, Mondini) in 4, Connexin/GJB2 in 5, auditory neuropathy in 2, Waardenburg Syndrome in 2, meningitis in 2, cytomegalovirus (CMV) in 1, and Usher Syndrome in 1. Participants were recruited from the Human Research Subjects Core database at Boys Town National Research Hospital (BTNRH). Children with significant intellectual disabilities, visual or motor delays were excluded from this study. All children were from homes where English was the primary language due to lack of availability of parallel standardized tests in languages other than English at the time of data collection.
Vestibular Function Testing:
Vestibular Evoked Myogenic Potential (VEMP):
Cervical and ocular VEMP (c- and oVEMP) were completed using an ICS Otometrics Chartr EP 200 system (Natus, Taastrup, DK). A 500 Hz Blackman-gated toneburst was presented monaurally via ER-3A insert earphones in rarefaction at 126 dB SPL (95 dB nHL) at a repetition rate of 5.1 per second (4 ms duration; 2 cycle rise/fall, 0 ms plateau). Electromyography (EMG) signals were amplified (5000 μv) and band-passed filtered 10–1000 Hz for cVEMP and 1–1000 Hz for oVEMP. One hundred sweeps were averaged for each cVEMP test and 200 sweeps were averaged for each oVEMP test. Each condition was replicated to ensure reproducibility.
For cVEMP testing, non-inverting electrodes were placed on each sternocleidomastoid (SCM) muscle with additional electrodes placed directly underneath the non-inverting electrode to measure EMG activity of the SCM, an inverting electrode was placed on the manubrium of the sternum, and a ground electrode was placed on the forehead. Electrode impedances were acceptable if they were under 5k ohms. Participants lay semi-recumbent, elevated 30 degrees. They were instructed to lift their head and turn away from the ear receiving acoustic stimulation. SCM EMG activity between 50–300 μV was necessary for each sweep. Participants were given a hand-held EMG monitor, which provided feedback of SCM contraction. cVEMP outcome variables were the p13 and n23 latencies (msec), p13/n23 raw amplitude (μV), and the corrected p13/n23 amplitude (raw p13/n23 amplitude divided by the average EMG).
For oVEMP testing, non-inverting electrodes were placed 3 mm below the margin of the lower eyelid, centered under the pupil, the inverting electrode was placed 2cm below the non-inverting electrode, and the ground electrode was placed on the forehead. Participants were instructed to look up 30° during acoustic stimulation. Responses were recorded from the eye contralateral to the stimulated ear. oVEMP outcome variables were the n10 and p16 latencies (msec), and the n10/p16 amplitude (μV).
Video Head Impulse Test (vHIT):
vHIT was measured with a computerized video head impulse system (Otometrics ICS Impulse, Schaumberg, IL). Participants were seated one meter from a visual target at eye level and were instructed to keep their eyes on the target as the clinician performed head impulses in the plane of each semicircular canal (right and left, horizontal, anterior and posterior). For each head impulse, the head was displaced 10 to 20° at a peak head velocity between 100 – 300°/s and peak head acceleration between 1000°/s2 and 2500°/s2. During head impulses, the infrared camera measured eye velocity while the gyroscope measured head velocity. The outcome parameter was gain, which was calculated by dividing eye velocity from head velocity.
Rotary Chair:
Rotary chair testing was completed in a motorized rotational chair (Micromedical Technologies, Chatham, IL). Sinusoidal harmonic acceleration (SHA) was completed at the following frequencies and velocities: 0.02 Hz (70°/sec), 0.08 Hz (50°/sec), 0.16 Hz (40°/sec), and 0.32 Hz (30°/sec). Nystagmus in response to rotation was measured with either an infrared video or electrodes. For each frequency of rotation, the outcome parameters were gain (eye velocity/chair velocity), phase, and symmetry.
Bruininks-Oseretsky Test of Motor Proficiency (BOT-2):
The BOT-2 is a standardized test of motor proficiency. Only the Balance subtest was completed. This subtest consists of 9 tasks which include: 1) standing with feet apart on a line, 2) walking on a line, 3) standing on one leg, 4) standing with feet apart on a line, eyes closed, 5) walking heel-to-toe, 6) standing on one leg, eyes closed, 7) standing on one leg, on a balance beam, 8) standing heel-to-toe on a balance beam, and 9) standing on one leg on a balance beam, eyes closed. If the maximum score is not achieved on the first trial, a second trial is attempted. Each task has a maximum score of 4, except for the 9th task, which has a maximum score of 5, for a total score possible of 37. A scaled score, based on age, was determined (range 1 – 35, mean 15, standard deviation 5; Bruininks & Bruininks, 2005).
Dynamic Visual Acuity:
Subjects were seated at eye level 12.5 feet from the computer monitor where visual targets were presented. Subjects who wore glasses or contact lenses were encouraged to wear them for testing. Visual targets were the letters C, D, H, K, O, N, S, R, V, A and Z, presented in random order. Letter were reviewed with the children prior to testing to ensure familiarity. Subjects wore a head mounted rate sensor positioned in the plane of the horizontal canal (O-Navi, Vista, CA, USA). Software was modified from the NIH Toolbox DVA test (Rine et al., 2012; Rine et al., 2013). Head velocity was detected by the rate sensor and visual targets were presented when head velocities were between 120–180 degrees/second. DVA was measured 4 times: in response to right- and leftward head movements both actively (with the subject shaking their own head) and passively (with the examiner moving the subject’s head).
Clinical measures:
A battery of clinical measures was administered to all participants assessing the following areas: working memory, executive function, hearing and speech perception, language, and vision. Working memory and executive function performance were the primary outcomes. Hearing, speech perception, language, and vision testing were completed to ensure that these factors were not driving group differences in the working memory and executive function outcomes, particularly in the group of children with vestibular loss. All clinical assessments were administered by an audiologist or a speech-language pathologist experienced in working with children with hearing loss. Administration of all assessments occurred in a quiet room and required 3–4 hours of testing over the course of 1–2 visits. CCI wore their cochlear implant (CI) during the testing sessions. Examiners followed standardized testing guidelines unless specified otherwise. Videotaped assessments were periodically reviewed to ensure proper adherence to protocols and assessments were double-scored and double-entered into a repository database to ensure accuracy.
Most participants used spoken English at home and in school; administration of their measures was in spoken language. Five participants used a combination of Signing Exact English (SEE-II) and spoken language in their educational settings and required varying levels of sign support for clinical measures in the individualized testing environment. Of these participants, two utilized an interpreter for most of the measures, which involved simultaneous presentation of items in sign (SEE-II) and spoken language. Two participants were provided sign support via an interpreter for test directions only; administration proceeded in spoken language. The remaining participant used the interpreter as needed, but not for all measures. The interpreter was fluent in SEE-II and served for several years as an educational interpreter in the schools. The research team developed guidelines for aligning Peabody Picture Vocabulary Test – 4 (PPVT-4) items with established SEE-II signs; items with no corresponding sign or items that were highly iconic were presented in fingerspelling. Finger-spelled items were also presented in print to ensure that measurement of vocabulary was not confounded by processing of fingerspelling. All remaining measures that involved sign support were presented in SEE-II to maintain English grammatical integrity. For all participants, speech perception testing was administered without sign support.
Background variables were gathered for each child with an intake interview. Parents or guardians were interviewed about demographic and family characteristics, childcare information, current services and health history, and hearing history. Medical records of the CCI were reviewed to obtain dates of implantation, etiologies of hearing loss and device types.
Working Memory:
The Automated Working Memory Assessment (AWMA; Alloway, 2007) was given to assess working memory in the domains of verbal short-term memory, verbal working memory, visuo-spatial short-term memory, and visuo-spatial working memory. Six subtests of the AWMA were given to participants: digit recall, counting recall, dot matrix, block recall, odd-one-out, and spatial recall. The digit recall subtest measures verbal short term memory. In digit recall, the child is required to recall a sequence of digits in the correct order. The number of digits in each series increases after the child attains four correct answers in a set of six sequences. The counting recall subtest measures verbal working memory and requires the child to count the number of red circles in an array of blue and red circles and triangles and then recall the correct number of red circles in an increasing sequence. The dot matrix and block recall subtests measure visuo-spatial short-term memory. In the dot matrix subtest, the child is required to watch the position of a red dot on a four by four grid and respond by indicating the dot’s position on a blank grid. The number of dots in each sequence increases when the child attains four correct answers in a set of six sequences. The block recall subtest requires the child to view a series of nine blocks being tapped and recall the sequence in the correct order by tapping on the image of the blocks. The number of blocks tapped in each sequence increase after the child attains four correct answers in a set of six sequences. The odd one out and spatial recall subtests measures visuo-spatial working memory. In the odd one out subtest the child is required to first indicate the “odd one out” or different shape from a set of three shapes and then to correctly recall the position of the different shape on an empty grid. The spatial recall subtest requires the child to determine if two shapes are the “same” or “opposite” of each other. The shape on the right has a red dot above it and may be rotated in reference to the shape on the left. The child then needs to recall the position of the red dot on an empty grid with three possible positions marked. Again, the number of grids or shapes in each sequence increases after the child obtains four correct answers in a set of six sequences. Results of each subtest are reported as raw scores.
Executive Function:
The Behavior Rating Inventory of Executive Function (BRIEF; Gioia, et. al., 2000) was given to assess parent report of executive functioning. The BRIEF is a parent-report questionnaire which measures the following domains of executive functioning; inhibit, shift, emotional control, initiate, working memory, plan/organize, organization of materials, and monitor. Broad indices of behavioral regulation and metacognition can be calculated along with the overall global executive composite score.
The Flanker Inhibitory Control and Attention (Flanker) and Dimensional Change Card Sort (DCCS) subtests of the NIH Toolbox (Gershon, et. al., 2013) were used to measure executive function. For each subtest, the age-corrected standard score is reported (mean = 100, SD = 15), which incorporates the child’s accuracy and reaction time. Attention and inhibitory control are measured by the Flanker subtest by requiring the child to focus on a stimulus while inhibiting attention to surrounding stimuli, both congruent and incongruent. Cognitive flexibility is measured by the DCCS subtest, which requires the child to match a series of bivalent pictures (e.g. yellow balls and blue trucks) to target pictures according to the dimensions of color and shape. Initially, trials are presented according to one dimension at a time, and then progress to alternating between dimensions to assess flexibility.
Hearing and Speech Perception:
Ear-specific audiometric thresholds for all CNH were obtained using conventional audiometry by an audiologist in a sound-treated, double-walled booth using insert or supra-aural headphones. CNH had air-conducted thresholds 15 dB HL or better at all octave frequencies 250–8000 Hz, bilaterally.
Speech perception was assessed using adult AZBio sentences (Spahr et al., 2012), or when the adult lists were too difficult for an older child or a child was under the age of 13 years, pediatric AZBio sentence lists were used (Spahr et al., 2014). The sentence lists were presented in the soundfield in quiet at 65 dB SPL. When requested, presentation level was increased to most comfortable loudness (MCL) for CCI. The number of words repeated correctly for each sentence was used to calculate a percent correct score for each participant.
Vocabulary:
Receptive vocabulary was assessed using the PPVT-4 (Dunn & Dunn, 2007), which requires the child to select the correct picture from a set of four choices when given a target word. Results are reported as standard scores calculated based on the child’s age.
Language sample:
Elementary age children (6–9 years) were also given a conversational language sample based on Pam Hadley’s interview protocol (1998) adapted from Dollaghan, Campbell, and Tomblin (1990), Evans and Craig (1992), and McCabe and Rollins (1994). The conversational interview included questions about the child’s family, favorite activities, and favorite books and movies. Participants aged 10–18 years were given an expository language sample which included a fable retell task. This language sample task was based on the interview protocol from the work of Nippold and colleagues (2008, 2014 & 2015) and included a conversational interview, expository interview about a favorite game or sport and fable retelling task followed by a series of critical thinking questions. The fable “The Fox and the Crow” was used for the retell portion of the language sample. Language samples were transcribed and coded for analysis in the Computerized Language Analysis (CLAN) program (MacWhinney, 2000). Mean length of utterance (MLU) was calculated for each participant. Reliability of language sample transcription was calculated on 10% of samples and word-by-word match of two transcribers was 93.93%.
Vision:
Each participant completed a visual acuity screening in a well-lit room using a Sloan letters chart (Ferris et al, 1982). Participants with prescription glasses or contacts were required to wear them during the screening. The non-test eye was occluded, and the participant identified the letters of each line down to the smallest line where 4 out of 5 letters could be correctly identified. A screening threshold of 20/32 or better in each eye was required to pass the screening.
Statistics:
To determine if there were significant differences between groups, a one-way ANOVA was computed for WASI-II, BOT-2 (raw and scaled scores), PPVT-4, Flanker, DCCS and BRIEF scores. When needed, post hoc testing was completed using Tukey’s honest significant difference (HSD). A between groups factorial ANOVA using raw score as the outcome variable and age as a covariate was computed for MLU and all AWMA subtests. When needed, post hoc testing was completed using Bonferroni correction. Multivariate regression analyses were completed for all working memory subtests to determine if average vHIT gain, age or PPVT-4 score predicted working memory performance. A secondary analysis was completed to determine if balance performance (BOT-2, scaled score) rather than vHIT gain better predicted working memory performance.
RESULTS:
Degree of vestibular loss:
All subjects completed vHIT, and rotary chair testing. All subjects except 1 with bilateral atresia completed VEMP testing. VEMP response rates and amplitudes are shown in Table 1 for CNH and CCI separated by implant ear (CCI-ipsi, n = 60) and non-implant ear (CCI-contra, n = 12). CNH demonstrated a significantly higher percentage of present cVEMP (X2 = 33.025, p < 0.001) and oVEMP (X2 = 25.482, p < 0.001) responses compared to CCI-ipsi and CCI-contra. There was not a significant difference in cVEMP response rates between CCI-ipsi and CCI-contra; however, CCI-ipsi demonstrated a significantly greater proportion of absent oVEMP responses compared to CCI-contra (p < 0.001). CNH and CCI-contra ears had significantly higher cVEMP amplitudes compared to CCI-ipsi (F = 34.479, p < 0.001); while the model was significant for oVEMP amplitude (F = 3.486, p = 0.033), post hoc group differences were not significant. Recent work in our lab showed that air-conducted VEMP testing may inaccurately depict the degree of vestibular loss in CCI (Merchant et al., 2020) due to the presence of air-bone gaps following cochlear implantation (Chole et al. 2014; Banakis Hartl et al. 2016; Mattingly et al. 2016). Response rates may be inaccurately absent in 26% of cVEMP and 37% of oVEMP responses (Merchant et al., 2020); therefore, degree of vestibular loss was defined by horizontal vHIT results and confirmed by rotary chair.
Table 1.
Cervical and Ocular VEMP Response Rates and Amplitudes
| CNH (n = 76) |
CCI | Statistic* | p-value | |||
|---|---|---|---|---|---|---|
| ipsi (n = 60) |
contra (n = 12) |
|||||
| Response Rate | 75/76 (99%) |
36/60 (60%) |
9/12 (75%) |
33.025 | < 0.001 | |
| Corrected Amplitude (SD) | 2.22 (1.08) |
0.71 (0.84) |
2.74 2.42) |
34.479 | < 0.001 | |
| Response Rate | 58/76 (76%) |
20/60 (33%) |
6/12 (50%) |
25.482 | < 0.001 | |
| Amplitude (SD) | 5.34 (5.64) |
2.97 (5.71) |
6.54 (8.17) |
3.486 | 0.033 | |
Response rate differences between CCI and CNH using Chi-Square and amplitude differences using one-way ANOVA
Head impulses were considered abnormal if gain was < 2 SDs below the normal control group mean (horizontal canal mean: 0.96, SD = 0.065, 2 SD = 0.82) and if compensatory saccades were present in greater than 80% of head impulses and exceeded 50° in amplitude (Janky et al., 2018b). This classification scheme resulted in the following subject groups: CNH (n = 38, mean age: 12.2 years, range 7 – 18), CCI with normal vestibular function (CCI-NV; n = 26, mean age: 13.5 years, range 7 – 19), and CCI with vestibular loss (CCI-VL; n = 11, mean age: 13, range 7 – 20). Of the CCI-VL, 5 had unilateral vestibular loss (CCI-UVL; mean age: 13.1, range 7 – 20) and 6 had bilateral vestibular loss (CCI-BVL; mean age: 13, range 7 – 19). Mean age at implantation was 39.9 months for CCI-NV, 28.6 months for CCI-UVL and 19.5 months for CCI-BVL.
All analyses include children with a WASI-II PRI composite score ≥ 77. There were no significant group differences in the WASI-II PRI composite score (F (3, 71) = .919, p = .436, ES = .19; Table 2). The 5 children who used sign support were spread across the CCI groups; 4 had normal vestibular function and 1 had unilateral vestibular loss.
Table 2.
Mean scores per group for all outcome measures.
| Outcomes | Group p-value |
Group | |||
|---|---|---|---|---|---|
| CNH | CCI-NV | CCI-UVL | CCI-BVL | ||
| Nonverbal Intelligence | |||||
| WASI-II PRI Composite Score | p = .436 | 109.3 | 104.4 | 101.2 | 105.7 |
| Balance Function | |||||
| BOT-2 – Raw Score | p < .001 | 32.5 | 30.6 | 24.4 | 20.2 |
| BOT-2 – Scaled Score | p < .001 | 13.43 | 10.85 | 6.2 | 4.6 |
| Dynamic Visual Acuity | |||||
| Active DVA | p = .003 | .23 | .23 | .30 | .41 |
| Passive DVA | p < .001 | .27 | .25 | .41 | .57 |
| Speech Perception | |||||
| AZBio | p < .001 | 99.6 | 86.5 | 74.2 | 87.7 |
| Language / Vocabulary | |||||
| PPVT-4 | p < .001 | 118.7 | 97.6 | 91.2 | 107.2 |
| MLU | p = .154 | 8.1 | 7.8 | 6.7 | 6.6 |
| Automated Working Memory Assessment | |||||
| Digit Recall* | p < .001 | 35.39 | 28.5 | 25.42 | 31.41 |
| Counting Recall* | p = .304 | 22.56 | 21.07 | 20.11 | 19.33 |
| Counting Recall Processing* | p = .348 | 77.01 | 68.32 | 67.03 | 59.37 |
| Dot Matrix* | p = .017 | 29.23 | 25.9 | 25.23 | 26.3 |
| Block Recall* | p = .210 | 26.52 | 25.22 | 23.99 | 22.56 |
| Odd One Out* | p = .087 | 27.18 | 24.92 | 25.27 | 22.66 |
| Odd One Out Processing* | p = .077 | 101.1 | 86.88 | 90.59 | 76.54 |
| Spatial Recall* | p = .715 | 24.48 | 22.64 | 25.06 | 22.49 |
| Spatial Recall Processing* | p = .522 | 89.22 | 76.06 | 89.8 | 72.88 |
| Executive Function | |||||
| Flanker | p = .004 | 114.34 | 94.09 | 99.56 | 97.98 |
| DCCS | p = .022 | 103.01 | 89.40 | 94.85 | 88.35 |
| BRIEF | p = .065 | 47.73 | 50.58 | 59.0 | 42.6 |
denotes univariate analyses completed; marginal means are reported.
Bold = significant group differences.
For the BOT-2, there was a significant difference in balance ability between groups using both the raw score (F (3, 67) = 22.4, p < .001, ES = .71) and scaled score (F (3, 67) = 11.37, p < .001, ES = .58). Post hoc testing for the raw score using Tukey’s HSD demonstrated no significant difference in mean performance between CNH and CCI-NV (p = .157), CCI-UVL performed significantly worse than CNH (p < 0.001), and CCI-BVL performed significantly worse than both CNH (p < .001) and CCI-NV (p < .001); there was no difference in BOT-2 performance between the groups of CCI with vestibular loss (p = .257, Table 2). Post hoc testing for the scaled score using Tukey’s HSD demonstrated a step-wise reduction in performance; CNH performed significantly better than both groups with vestibular loss (UVL, p = .01, BVL, p = < .001), CCI-NV performed better than CCI-BVL (p = .009), and there was no difference between the groups of CCI with vestibular loss (p = .916, Table 2).
There was a significant difference in DVA between groups both actively (F (3, 64) = 5.013, p = .003, ES = .44) and passively (F (3, 63) = 9.556, p < .001, ES = .56); see Table 2. Post hoc testing using Tukey’s HSD demonstrated that for both active and passive DVA, CCI-BVL had significantly worse DVA scores compared to CNH and CCI-NV (p = .005 - < .001). There was no difference between the groups of CCI with vestibular loss (active: p = .381, passive: p = .221).
Speech perception (AZBio) and language (PPVT-4 & a language sample) evaluations were completed. As expected, there were significant group differences in AZBio performance (F (3, 71) = 6.75, p < .001, ES = .47) and PPVT-4 (F (3, 71) = 11.52, p < .001, ES = .57). For both outcomes CNH outperformed all CCI except for CCI-BVL; there were no significant differences between CCI-NV, CCI-UVL and CCI-BVL (Table 2). For MLU on the language sample, while age was a significant covariate (F (1, 62) = 9.88, p = .003, ES = .57), there was no significant difference in performance between groups (F (3, 62) = 2.63, p = .058, ES = .34).
Automated Working Memory Assessment (AWMA):
For digit recall, a measure of verbal short-term memory, significant group differences in performance were noted (F (3, 65) = 7.27, p < .001, ES = .5, Figure 1A) with age as a significant covariate (F (1, 65) = 7.26, p = .009). Post hoc testing revealed that CNH performed better than both CCI-NV (p = .001) and CCI-UVL (p = .01). There were no significant differences between the CCI groups.
Figure 1. Estimated Marginal Means by group for each Automated Working Memory Assessment subtest.




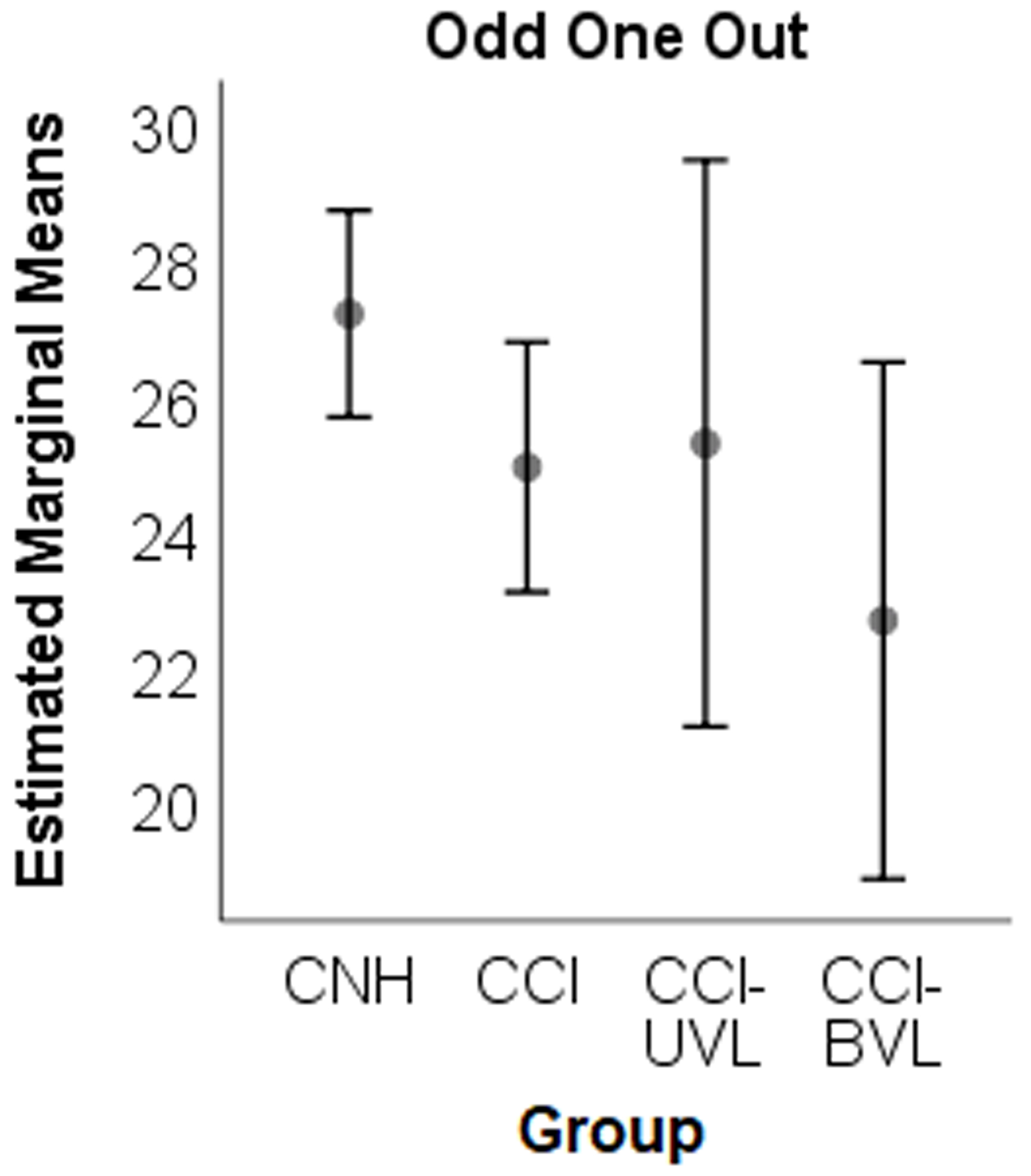

There was no significant reduction in working memory performance for either group of CCI with vestibular loss. CNH – children with normal hearing; CCI – children with cochlear implants, UVL = unilateral vestibular loss, BVL = bilateral vestibular loss.
For counting recall, a measure of verbal working memory, there was no significant difference in performance between groups (F (3, 69) = 1.24, p = .304, ES = .23, Figure 1B); however, age was a significant covariate (F (1, 69) = 25.22, p < .001). Similar findings were noted for counting recall processing. There was no significant difference between groups (F (3, 69) = 1.12, p = .348, ES = .22); however, age was a significant covariate (F (1, 69) = 17.4, p < .001).
For measures of visuo-spatial short-term memory, there were significant group differences in performance for dot matrix (F (3, 70) = 3.63, p = 0.017, ES = .37, Figure 1C), but not block recall (F (3, 67) = 1.55, p = .21, ES = .25, Figure 1D) with age as a significant covariate for both dot matrix (F (1, 70) = 59.92, p < .001) and block recall (F (1, 67) = 31.46, p < .001). For the dot matrix subtest, post hoc testing revealed that CNH performed better than both CCI-NV (p = .009) and CCI-UVL (p = .048). There were no significant differences between the CCI groups.
For measures of visuo-spatial working memory, there were no significant differences in performance between groups for odd-one-out (F (3, 70) = 2.28, p = .087, ES = .3, Figure 1E), odd-one-out processing (F (3, 70) = 2.38, p = .077, ES = .3), spatial recall (F (3, 70) = .46, p = .715, ES = .14, Figure 1F), or spatial recall processing (F (3, 70) = .76, p = .522, ES = .18). Age was a significant covariate for odd-one-out (F (1, 70) = 40.74, p < .001), odd-one-out processing (F (1, 70) = 38.21, p < .001), spatial recall (F (1, 70) = 18.32, p < .001), and spatial recall processing (F (1, 70) = 17.23, p < .001).
In summary, there was no significant reduction in working memory performance for either group of CCI with vestibular loss on the AWMA tasks. While no differences were noted in the performance of CCI compared to CNH on any of the verbal or visuospatial working memory tasks, CCI-NV and CCI-UVL groups performed significantly poorer than CNH on the verbal short-term memory subtest and one of the visuospatial short-term memory subtests. Given the small sample size, multivariate regression analysis was completed to determine if average vHIT gain, age or PPVT-4 vocabulary score predicted AWMA performance. A secondary analysis was completed to determine if balance performance (BOT-2, Scaled Score) or passive DVA better predicted AWMA performance. Models with higher order interactions were not significant; therefore, only the main effects are reported in Table 3. As expected, age was a significant predictor for all AWMA subtests, while PPVT-4 was a significant predictor for all AWMA subtests except for Block Recall and Odd One Out. Average vHIT gain and passive DVA were not predictors of any working memory subtest (Table 3); however, interestingly BOT-2 performance (Scaled Score) was a significant predictor for several AWMA subtests (Table 3, Figure 2A–F).
Table 3:
Multivariate Regression analyses
| Outcome | R2 | Predictor Variables(Unstandardized B) | R2 | Predictor Variables(Unstandardized B) | R2 | Predictor Variables(Unstandardized B) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| age | PPVT-4 | vHIT | age | PPVT-4 | BOT-2 | age | PPVT-4 | DVA | ||||
| Automated Working Memory Assessment | ||||||||||||
| Digit Recall | .329 | .72 | .22 | −.56 | .398 | .81 | .20 | .32 | .368 | .75 | .23 | −4.41 |
| Counting Recall | .289 | .82 | .08 | 1.53 | .377 | .91 | .07 | .28 | .328 | .87 | .10 | −2.7 |
| Counting Recall Processing | .226 | 3.87 | .43 | 4.05 | .313 | 4.23 | .35 | 1.63 | .265 | 4.07 | .50 | −21.31 |
| Dot Matrix | .415 | 1.09 | .07 | 1.02 | .453 | 1.12 | .06 | .23 | .411 | 1.09 | .07 | .32 |
| Block Recall | .329 | .91 | .05 | 5.74 | .320 | .86 | .03 | .25 | .280 | .80 | .05 | −4.27 |
| Odd One Out | .35 | 1.00 | .05 | 4.12 | .395 | 1.03 | .03 | .29 | .354 | 1.03 | .06 | −.21 |
| Odd One Out Processing | .329 | 5.47 | .3 | 18.22 | .369 | 5.61 | .22 | 1.47 | .340 | 5.67 | .37 | 9.35 |
| Spatial Recall | .274 | 1.17 | .13 | 1.03 | .300 | 1.16 | .14 | −.07 | .306 | 1.21 | .15 | .28 |
| Spatial Recall Processing | .273 | 6.24 | .78 | 9.83 | .307 | 6.35 | .82 | −.53 | .303 | 6.54 | .83 | −.49 |
| Executive Function | ||||||||||||
| Flanker | .301 | −.801 | −.037 | −7.78 | .320 | −.957 | −.053 | −.185 | .377 | −.91 | −.05 | 11.65 |
| DCCS* | .530 | −3.02 | −1.233 | −60.72 | .366 | −4.2 | −.652 | .716 | .289 | −3.93 | −.42 | 47.63 |
| BRIEF | .061 | .040 | −.117 | 8.297 | .041 | .127 | −.100 | .009 | .054 | −.01 | −.13 | −1.09 |
PPVT-4 = Peabody Picture Vocabulary Test; vHIT = video head impulse test; BOT-2 = Bruininks-Oseretsky Test of Motor Proficiency. DVA = Dynamic Visual Acuity. All significant effects are bolded,
unstandardized B coefficients from the model with 2-way interactions.
Figure 2. Scatterplots of Automated Working memory performance for each subtest by BOT-2 Scaled Score.






Average vHIT gain was not a predictor of any working memory subtest; however, the BOT-2 Scaled Score was a significant predictor for several working memory subtests. * = subtests in which BOT-2 performance was a significant predictor.
Executive Function:
For the Flanker, a measure of attention and inhibitory control, significant group differences in performance (F (3, 68) = 4.93, p = 0.004, ES = .42, Table 2) were noted. Post hoc testing revealed that CCI-NV performed significantly more poorly than CNH (p = .003). There was no statistically significant difference in performance between CNH and either group of CCI with vestibular loss (UVL or BVL).
For the DCCS, a measure of cognitive flexibility, significant group differences in performance (F (3, 70) = 3.41, p = 0.022, ES = .36, Table 2) were noted. Like Flanker performance, post hoc testing revealed that CCI-NV performed significantly more poorly than CNH (p = .022). There was no statistically significant difference in performance between CNH and either group of CCI with vestibular loss (UVL or BVL).
For the BRIEF, a parent report of executive functioning, there was no significant difference in performance between groups for the global composite score (F (3, 66) = 2.52, p = .065, ES = .32, Table 2).
In summary, there was no significant reduction in executive function performance for either group of CCI with vestibular loss compared to CNH or CCI-NV on the executive function tasks; however, CCI-NV performed significantly poorer than CNH on the Flanker and DCCS. Given the small sample size, multivariate regression analysis was completed to determine if average vHIT gain, age or PPVT-4 vocabulary score predicted executive function performance. A secondary analysis was completed to determine if balance performance (BOT-2, Scaled Score) or passive DVA better predicted executive function performance. Models with higher order interactions were not significant for the Flanker or BRIEF; therefore, only the main effects are reported in Table 3. For the Flanker, age and vHIT, but not PPVT-4, were significant predictors. In the secondary analysis with balance, only age was a significant predictor. In the analysis with passive DVA, both age and passive DVA were predictors. For the DCCS, the two-way interactions between PPVT-4 and age and PPVT-4 and vHIT gain were significant predictors, indicating the relationship between DCCS and PPVT-4 is dependent on both age and vHIT gain. In the secondary analysis, only the two-way interaction between PPVT-4 and age were significant predictors for both balance and passive DVA. For the BRIEF, none of the models were significant.
DISCUSSION
The purpose of the current study was to quantify degree of vestibular loss in CCI and evaluate the effect of vestibular loss on working memory and executive function compared to CNH. Overall, our results show there was no significant effect of vestibular loss on working memory or executive function performance. The findings of the current study indicate that hearing loss, and not vestibular loss, had a detrimental effect on the executive function abilities of the children tested. No group differences in the Flanker, DCCS or BRIEF results were noted between the groups of CCI. Our findings are consistent with Bigelow et al. (2015) who found that tests of executive function and verbal memory were not associated with vestibular loss. While this may seem to contradict the findings of Popp et al. (2017) who found that executive function was significantly affected in adults with vestibular loss; hearing loss was not a factor considered in the study. vHIT gain was found to be a significant predictor of the Flanker, but not all tests of executive function. This disparate finding could reflect the association between vestibular loss and subsequent deficits in attention (Bigelow et al., 2015; Dobbels et al., 2019).
For working memory performance, we found no group differences for any of the AWMA subtests with respect to CCI with vestibular loss. Further, neither average vHIT gain nor passive DVA were significant predictors of any working memory subtest; however, balance ability (i.e., BOT-2 Scaled Score) was a significant predictor for several working memory subtests. Differences in our outcomes regarding working memory compared to others (Lacroix et al. 2020; Guidetti et al, 2020; Bigelow et al., 2015; Popp et al., 2017) could be related to several factors.
First, the vestibular system is comprised of 5 different rate sensors. Thus, there are various vestibular assessment methods that can be used to quantify vestibular function (i.e., caloric, rotary chair, vHIT, cervical VEMP, ocular VEMP, etc). There has been wide variation among studies in the vestibular function test used to group subjects. Some have used cervical VEMPS in isolation (Bigelow et al., 2015), at least one abnormality among cervical VEMP, ocular VEMP and caloric (Lacroix et al., 2020), a combination of several outcomes (vHIT, caloric, rotary chair, cervical VEMP, Dobbels et al., 2019; Xie et al., 2017), history of bilateral nerve section (Brandt et al., 2005), or not fully disclosing the method of assessing vestibular function (Guidetti et al., 2020). In the current study, vHIT was used to quantify degree of vestibular loss. While it has been estimated that approximately 50% of CCI evidence some degree of vestibular loss with 50% of those having BVL (Cushing et al., 2013), only 11/37 (30%) in the current study had vestibular loss; of those 5/11 (45%) had unilateral vestibular loss and 6/11 (55%) had bilateral vestibular loss. This rate of vestibular loss is lower than what has been reported in the literature. We attribute our lower rate of vestibular loss to not using VEMP data to quantify degree of vestibular loss. Air-conducted VEMPs were completed on all participants (Table 1); however, after data collection was completed for the present study our lab demonstrated that air-conducted VEMP testing may inaccurately depict the degree of vestibular loss in CCI (Merchant et al., 2020). Placement of a cochlear implant has been shown to result in small air-bone gaps (Chole et al. 2014; Banakis Hartl et al. 2016; Mattingly et al. 2016). While this is not relevant to the hearing of most children post-CI as they are stimulated electrically via the implant, we showed that compared to air-conduction, VEMP response rates improved when using bone conduction suggesting this air-bone gap can abolish the air-conducted VEMP (Merchant et al., 2020). Therefore, VEMP data was not used to quantify degree of vestibular loss. Use of vHIT in isolation could have resulted in an underestimation of the degree of vestibular loss in the current study. Additionally, there are conflicting findings regarding the links between cognitive deficits and otolith function (Bigelow et al., 2015; Dobbels et al., 2019). As noted, otolith function was not considered in this study, which could be a focus of future work in children.
Second, few studies have considered the additional effect of hearing loss on cognitive outcomes. Lacroix et al. (2020) noted reduced performance on measures of visuospatial working memory, mental rotation and space orientation, but not visual attention; however, their children represented a small (n = 13) heterogeneous cohort with varying degree and type of vestibular loss as well as hearing loss. Vestibular loss was quantified as at least one abnormality among cervical VEMP, ocular VEMP and caloric (Lacroix et al., 2020), suggesting a heterogeneous cohort regarding vestibular loss type (otolith versus canal) and severity. Additionally, 7/13 subjects had hearing loss necessitating either a hearing aid or a cochlear implant and the remaining children had normal hearing suggesting a heterogeneous cohort regarding hearing loss severity, which was not factored into any analyses. Dobbels et al. (2019) specifically investigated the effect of both vestibular and hearing loss on cognition and found that hearing loss was associated with deficits in immediate memory and language, while vestibular loss was associated with deficits in attention. In the current study, 2 control groups were included, an age-matched group with normal hearing and an age-matched group matched on degree of hearing loss (i.e., speech perception) and vocabulary, but normal vestibular function. While performance on the working memory outcomes demonstrated a downward trend as vestibular loss severity increased (Fig 1A–F), reduced performance can also be noted for the children with hearing loss and normal vestibular function, suggesting that hearing loss alone, and not vestibular loss, plays a significant role in working memory performance, which is a consistent finding across studies in children with hearing loss who utilize a CI (Nittrouer et al., 2013; Nittrouer et al., 2017). Significant differences were also noted between our control population with normal hearing and CCI with normal vestibular function on tests of executive function, which is also consistent with others (Beer et al., 2014). Without the inclusion of the control group with hearing loss and normal vestibular function, significant differences in performance between CCI with vestibular loss and CNH may have been misattributed to vestibular loss. Instead, the results suggest that hearing loss is a significant consideration when investigating the effects of vestibular loss on cognition.
Third, while there was not a statistically significant difference in AWMA or executive function performance between CCI-NV and CCI-UVL and -BVL, Figure 1A–F demonstrates a downward trend in performance in children with vestibular loss for the AWMA and Table 2 shows that CCI-BVL had the lowest mean DCCS scores, yet did not perform significantly different from the CNH group. Effect sizes varied from 0.14 – 0.5. This coupled with our small sample size of children with vestibular loss (n = 11) suggest that our findings could be underpowered. Despite our small sample size, separating the children with UVL from those with BVL was felt to be necessary given cognitive deficits in both animals and humans are tied with degree of vestibular involvement; those with BVL demonstrating more severe deficits than those with UVL (Grabherr et al., 2011; Péruch et al., 2011; Popp et al., 2017). Initial analyses were completed combining the children with UVL and BVL and our pattern of findings remained the same. Additionally, it could be reasonably justified to include several factors that might affect working memory or executive function performance in addition to vestibular loss, but we are not adequately powered to include all these variables in any given model. Thus, we attempted to match the groups on nonverbal intelligence, speech perception and language/vocabulary. While there were no statistically significant differences between CCI groups for all these measures, Table 2 demonstrates some potential clinical differences, further suggesting that our findings could be underpowered. The groups appear to be balanced with respect to age, use of sign support and those who had unilateral versus bilateral CI (Table 4). While the distribution of household income was also similar among the groups (X2 = 22.84, p = 0.088), there was a significant difference in maternal education (X2 = 22.949, p = 0.006). The CNH tended to have mothers with a college or postgraduate education compared to CCI-NV whose maternal education was evenly distributed, which could account for some of the group differences between CNH and CCI-NV.
Table 4.
Demographic Data
| Group | % use sign support | Mean Age (range) |
% bilateral CI | Household Income | Mother’s Education | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 20 – 40k | 40 – 60k | 60 – 80k | 80 – 100k | > 100k | NR | HS | PS | C | PG | ||||
|
CNH
N = 38 |
0% | 12.2 years (7 – 18) |
0% | 0% | 3% | 5% | 16% | 55% | 21% | 0% | 3% | 43% | 54% |
|
CCI-NV
N = 26 |
15% | 13.5 years (7 – 19) |
69% | 23% | 12% | 15% | 4% | 23% | 23% | 27% | 19% | 19% | 35% |
|
CCI-UVL
N = 5 |
20% | 13.1 years (7 – 20) |
60% | 20% | 0% | 20% | 20% | 20% | 20% | 0% | 20% | 60% | 20% |
|
CCI-BVL
N = 6 |
0% | 13 years (7 – 19) |
67% | 0% | 17% | 0% | 17% | 50% | 17% | 0% | 17% | 33% | 50% |
HS = Completed High School, PS = Postsecondary Education, C = College Graduate, PG = Postgraduate work
Of the children with vestibular loss, all had deficits in balance performance on the BOT-2. CNH and CCI-NV were all within 1 SD of the mean (Scaled score mean = 15, SD = 5); however, both groups of CCI with vestibular loss scored greater than 1 SD below the mean with CCI-BVL scoring greater than 2 SDs below the mean. In addition to age, and in some instances vocabulary, balance performance was found to be a significant predictor of several working memory subtests (Table 3). Postural control has been shown to be related to cognitive and academic outcomes. Greater postural sway has been documented in children who were underperforming in school (Tomaz et al., 2014). Balance skills have been shown to account for a significant amount of the variance in both spatial and proportional reasoning (Frick et al., 2016). In adults with vestibular loss, posture and short-term memory are significantly associated (Smith et al., 2019). Our results suggest that postural control accounts for some of the variance in working memory performance as well. Because balance function improves with vestibular rehabilitation (Rine et al., 2004; Lofti et al., 2017), studies investigating the relationship between cognitive deficits and balance performance as well as the effects of vestibular rehabilitation on cognitive deficits could be a focus of future work in children with hearing and vestibular loss.
Of the children with vestibular loss, only CCI-BVL had significant deficits in DVA. DVA was not a significant predictor of any working memory or executive function subtests following a similar pattern to average vHIT gain suggesting these two tests reflect similar underlying constructs.
While the pathophysiology underlying cognitive deficits in individuals with vestibular loss is unknown, there are several hypotheses. The vestibular system has numerous projections to the insula, superior temporal gyrus, hippocampus, thalamus, cerebellum and the inferior parietal lobule, among other cortical areas (e.g., Bigelow & Agrawal, 2015, Hitier et al., 2014) which respond to vestibular stimulation (e.g., Hanes & McCollum, 2006). Additionally, there is likely an interaction among these areas (Hitier et al., 2014). Vestibular information is thought to modulate place, border, head direction, and grid cells in the hippocampus; thus, accounting for some of the cognitive changes in individuals with vestibular loss (Hitier et al., 2014). Brandt et al. (2005) noted decreased hippocampus volume in patients with bilateral vestibular loss which was linked to spatial memory deficits. This finding was later replicated by Kremmyda et al. (2016) in patients with partial bilateral vestibular loss. However, differences in hippocampal atrophy have been noted between human and animal studies. Because stress and anxiety are higher in individuals with vestibular loss (Guinand et al., 2012; Ayar et al., 2020), an interaction between hippocampal volume, vestibular loss, and stress/anxiety has been proposed (Kremmyda et al., 2016) and a ‘vestibular cognitive affective syndrome’ has been proposed to account for the constellation of cognitive, psychiatric and sleep symptoms (Smith et al., 2019). In children, the hippocampus undergoes several stages of maturation, thus, early onset vestibular loss is hypothesized to affect cognitive performance (Wiener-Vacher et al., 2013); however, few studies have investigated cognitive function in children with vestibular loss along with the concurrence of other psychiatric, sleep or fatigue symptoms.
Because our study population consisted of children, our results may deviate from those of others in that they do not account for the aging process or physiological changes that occur in the event vestibular loss is acquired. In children with hearing and vestibular loss, vestibular loss is either congenital or early acquired. While we hypothesize that the consequences of vestibular loss are the same in children as they are in adults, it may be that early onset vestibular loss has a differential effect on children given the time course within development. Future studies are needed to better understand the interaction of hearing loss and vestibular loss severity on various cognitive outcomes in children with early onset hearing and vestibular loss.
CONCLUSIONS:
Overall, results suggest that vestibular loss does not play a significant role in the working memory or executive function abilities of children with hearing and vestibular loss; however, balance ability accounted for a significant part of the variance in working memory performance. Our results may vary from others in our lack of including tests of otolith function and due to our small sample size. Children with hearing loss are a heterogenous group with varying degree of vestibular loss, degree of hearing loss, use of sign support, speech perception, and language ability. Thus, further investigations assessing the effects of vestibular loss on cognition in children should incorporate tests of both canal and otolith function, larger sample sizes and should control for additional variables which could drive group level effects such as degree of hearing loss, use of sign support, speech perception, and language ability; not accounting for these factors could result in group differences misattributed to vestibular loss.
Acknowledgements:
The authors would like to thank Meggie Dallapiazza for her work collecting preliminary cognitive data, which was not published as part of this manuscript, but lead to the eventual data collected within this manuscript. We would also like to thank Kendra Schmid for her consultation regarding statistical analyses.
Source of Funding:
Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under award number P20GM109023 and by the National Institute on Deafness and Other Communication Disorders under award numbers R03DC015318 and P30DC004662.
Footnotes
Conflicts of Interest:
KLJ provided consulting for Otometrics regarding the clinical use of vestibular evoked myogenic potential testing and video head impulse testing (vHIT) during this time frame.
Reference List
- [1].Alloway TP. Automated Working Memory Assessment. Pearson Assessment, London, 2007. [Google Scholar]
- [2].Ayar DA, Kumral E, and Celebisoy N. Cognitive functions in acute unilateral vestibular loss. J Neurol (2020), online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Banakis Hartl RM, Mattingly JK, Greene NT, Jenkins HA, Cass SP, and Tollin DJ. A preliminary investigation of the air-bone gap: Changes in intracochlear sound pressure with air- and bone-conducted stimuli after cochlear implantation. Otol Neurotol 37 (2016), 1291–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bigelow RT, Semenov YR, Trevino C, Ferrucci L, Resnick SM, Simonsick EM, Xue QL, and Agrawal Y. Association between visuospatial ability and vestibular function in the Baltimore Longitudinal Study of Aging. J Am Geratr Soc 63(9) (2015), 1837–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Beer J, Kronenberger WG, Castellanos I, Colson BG, Henning SC, and Pisoni DB. Executive functioning skills in preschool-age children with cochlear implants. J Speech Lang Hear Res 57(4) (2014), 1521–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Brandt T, Schautzer F, Hamilton DA, Bruning R, Markowitsch HJ, Kalla R, Darlington C, Smith P, and Strupp M. Vestibular loss causes hippocampal atrophy and impaired spatial memory in humans. Brain 128 (2005), 2732–2741. [DOI] [PubMed] [Google Scholar]
- [7].Braswell J, and Rine RM. Evidence that vestibular hypofunction affects reading acuity in children. Int J Pediatr Otorhinolaryngol 70 (2006), 1957–1965. [DOI] [PubMed] [Google Scholar]
- [8].Bruininks RH, and Bruininks BD BD. BOT2: Bruininks-Oseretsky Test of Motor Proficiency, 2nd ed. Pearson, 2005. [Google Scholar]
- [9].Chole RA, Hullar TE, and Potts LG. Conductive component after cochlear implantation in patients with residual hearing conservation. Am J Audiol 23 (2014), 359–364. [DOI] [PubMed] [Google Scholar]
- [10].Cushing SL, Gordon KA, Rutka JA, James AL, and Papsin BC. Vestibular end-organ dysfunction in children with sensorineural hearing loss and cochlear implants: An expanded cohort and etiologic assessment. Otol Neurotol 34(3) (2013), 422–8. [DOI] [PubMed] [Google Scholar]
- [11].Dobbels B, Mertens G, Gilles A, Claes A, Moyaert J, Van De Berg R, Van de Heyning P, Vanderveken O, and Van Romaey V. Cognitive function in acquired bilateral vestibulopathy: A cross-sectional study on cognition, hearing, and vestibular loss. Front Neurosci 13 (2019), 340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Dollaghan C, Campbell T, and Tomblin R. Video narration as a language sampling context. Journal of Speech and Hearing Disorders 55 (1990), 582–590. [DOI] [PubMed] [Google Scholar]
- [13].Dunn LM, and Dunn DM. Peabody Picture Vocabulary Test (4th ed.). American Guidance Service, Circle Pines, MN, 2007. [Google Scholar]
- [14].Evans J, and Craig H. Language sample collection and analysis: Interview compared to free play assessment contexts. J Speech Hear Res 35 (1992), 343–353. [DOI] [PubMed] [Google Scholar]
- [15].Ferris FL, Kassoff A, Bresnick GH, and Bailey I. New visual acuity charts for clinical research. Am J Ophthalmol, 94(1) (1982), 91–96. [PubMed] [Google Scholar]
- [16].Franco ES, and Panhoca I. Otoneurologic evaluation in children with school difficulties: Vestibular function investigation. Rev Bras Otorinolaringol 73(6) (2007), 803–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Franco ES, and Panhoca I. Vestibular function in children underperforming at school. Rev BrasOtorrinolaringol 74(6) (2008), 8158–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Frick A, and Möhring W. A matter of balance: Motor control is related to children’s spatial and proportional reasoning skills. Front Psychol 6 (2016), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gershon RC, Wagster MV, Hendrie HC, Fox NA, Cook KF, Nowinski CJ. NIH toolbox for assessment of neurological and behavioral function. Neurology 80 (11 Suppl 3) (2013), S2–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Gioia GA, Isquith PK, Guy SC, and Kenworthy L. Behavior Rating Inventory of Executive Function. Psychological Assessment Resources, Inc, Lutz, FL, 2000. [Google Scholar]
- [21].Grabherr L, Cuffel C, Guyot JP, and Mast FW. Mental transformation abilities in patients with unilateral and bilateral vestibular loss. Exp Brain Res 209 (2011), 205–214. [DOI] [PubMed] [Google Scholar]
- [22].Guidetti G, Guidetti R, Manfredi M, and Manfredi M. Vestibular pathology and spatial working memory. Acta Otorhinolaryngol Ital 40(1) (2020), 72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Guinand N, Boselie F, Guyot JP, and Kingma H. Quality of life of patients with bilateral vestibulopathy. Ann Otol Rhinol Laryngol 121 (2012), 471–477. [DOI] [PubMed] [Google Scholar]
- [24].Hanes DA, and McCollum G. Cognitive-vestibular interactions: A review of patient difficulties and possible mechanisms. J Vest Res 16 (2006), 75–91. [PubMed] [Google Scholar]
- [25].Hadley PA. Language sampling protocols for eliciting text-level discourse. Language, Speech, and Hearing Services in Schools 29 (1998), 132–147. [DOI] [PubMed] [Google Scholar]
- [26].Hitier M, Besnard S, and Smith PF. Vestibular pathways involved in cognition. Front Integr Neurosci 8 (59) (2014), 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Inoue A, Iwasaki S, Ushio M, Chihara Y, Fujimoto C, Egami N, and Yamasoba T. Effect of vestibular dysfunction on the development of gross motor function in children with profound hearing loss. Audiol Neurotol 18 (2013), 143–151. [DOI] [PubMed] [Google Scholar]
- [28].Janky KL, and Givens D. Vestibular, visual acuity and balance outcomes in children with cochlear implants: a preliminary report. Ear Hear 36(6) (2015), 364–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Janky KL, Thomas MLA, High RR, Schmid KK, and Ogen A. Predictive factors for vestibular loss in children with hearing loss. Am J Audiol 27(1) (2018a), 137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Janky KL, Patterson JN, Shepard NT, Thomas MLA, Barin K, Cruetz T, Schmid K, and Honaker JA (2018b). Video head impulse test (vHIT): The role of corrective saccades in identifying patients with vestibular loss. Otol Neurotol, 39(4), 467–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kimura Y, Masuda R, and Kaga K. Vestibular function and gross motor development in 195 children with congenital hearing loss – Assessment of inner ear malformations. Otol Neurotol 39 (2017), 196–205. [DOI] [PubMed] [Google Scholar]
- [32].Kremmyda O, Hüfner K, Flanagin V, Hamilton DA, Linn J, Strupp M, Jahn K, and Brandt T. Beyond dizziness: Virtual navigation, spatial anxiety and hippocampal volume in bilateral vestibulopathy. Front Hum Neurosci 10 (139) (2016), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lacroix E, Edwards MG, De Volder A, P Noel M, Rombaux P, and Deggouj N. Neuropsychological profiles of children with vestibular loss. J Vestib Res 30(1) (2020), 25–33. [DOI] [PubMed] [Google Scholar]
- [34].Li CM, Hoffman HJ, Ward BK, Cohen HS, and Rine RM. Epidemiology of dizziness and balance problems in children in the United States: A population-based study. J Pediatr 171 (2016), 240–247. [DOI] [PubMed] [Google Scholar]
- [35].Lotfi Y, Rezazadeh N, Moossavi A, Haghgoo HA, Rostami R, Bakhshi E, Badfar F, Moghadam SF, Sadeghi-Firoozabadi V, and Khodabandelou Y. Preliminary evidence of improved cognitive performance following vestibular rehabilitation in children with combined ADHD (cADHD) and concurrent vestibular impairment. Auris Nasus Larynx 44 (2017), 700–707. [DOI] [PubMed] [Google Scholar]
- [36].MacWhinney B. The CHILDES Project: Tools for Analyzing Talk (3rd ed.). Lawrence Erlbaum Associates, Mahwah, NJ, 2000. [Google Scholar]
- [37].Martin W, Jelsma J, and Rogers C. Motor proficiency and dynamic visual acuity in children with bilateral sensorineural hearing loss. Int J Pediatr Otorhinolaryngol 76(10) (2012), 1520–1525. [DOI] [PubMed] [Google Scholar]
- [38].Mattingly JK, Uhler KM, and Cass SP. Air-bone gaps contribute to functional hearing preservation in cochlear implantation. Otol Neurotol 37 (2016), 1255–1262. [DOI] [PubMed] [Google Scholar]
- [39].McCabe A, and Rollins P. Assessment of preschool narrative skills. Am J Speech Lang Pathol 3(1) (1994), 45–56. [Google Scholar]
- [40].Merchant GR, Schulz KM, Patterson JN, Fitzpatrick D, and Janky KL. Effect of cochlear implantation on vestibular evoked myogenic potentials and wideband acoustic immittance. Ear Hear (2020), online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Nippold MA, Mansfield TC, Billow JL, and Tomblin JB. Expository discourse in adolescents with language impairments: Examining syntactic development. Am J Speech Lang Pathol 17 (2008), 356–366. [DOI] [PubMed] [Google Scholar]
- [42].Nippold MA, Frantz-Kaspar MW, Cramond PM, Kirk C, Hayward-Mayhew C, and MacKinnon M. Conversational and narrative speaking in adolescents: Examining the use of complex syntax. J Speech Lang Hear Res 57 (2014), 876–886. [DOI] [PubMed] [Google Scholar]
- [43].Nippold MA, Frantz-Kaspar MW, Cramond PM, Kirk C, Hayward-Mayhew C, and MacKinnon M. Critical thinking about fables: Examining language production and comprehension in adolescents. J Speech Lang Hear Res 58 (2015), 325–335. [DOI] [PubMed] [Google Scholar]
- [44].Nittrouer S, Caldwell-Tarr A, Low KE, and Lowenstein JH. Working memory in children with cochlear implants: Problems are in storage, not processing. Int J Pediatr Otorhinolaryngol 77(11) (2013), 1886–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Nittrouer S, Caldwell-Tarr A, Low KE, and Lowenstein JH. Verbal working memory in children with cochlear implants. J Speech Lang Hear Res 60(11) (2017), 3342–3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].O’Reilly RC, Morlet T, Nicholas BD, Josephson G, Horlbeck D, Lundy L, and Mercado A. Prevalence of vestibular and balance disorders in children. Otol Neurotol 31 (2010), 1441–1444. [DOI] [PubMed] [Google Scholar]
- [47].Péruch P, Lopez C, Redon-Zouiteni C, Escoffier G, Zeitoun A, Sanjuan M, Devéze A, Magnan J, and Borel L. Vestibular information is necessary for maintaining metric properties of representational space: evidence from mental imagery. Neuropsychologia 11 (2011), 3136–44. [DOI] [PubMed] [Google Scholar]
- [48].Popp P, Wulff M, Finke K, Ruhl M, Brandt T, and Dieterich M. Cognitive deficits in patients with chronic vestibular failure. J Neurol 264 (2017), 554–563. [DOI] [PubMed] [Google Scholar]
- [49].Rine RM, Cornwall G, Gan K, LoCascio C, O’Hare T, Robinson E, and Rice M. Evidence of progressive delay of motor development in children with sensorineural hearing loss and concurrent vestibular dysfunction. Percept Mot Skills 90 (2000), 1101–1112. [DOI] [PubMed] [Google Scholar]
- [50].Rine RM, and Braswell J. A clinical test of dynamic visual acuity for children. Int J Pediatr Otorhinolaryngol 67 (2003), 1195–201. [DOI] [PubMed] [Google Scholar]
- [51].Rine RM, Braswell J, Fisher D, Joyce K, Kalar K, and Shaffer M. Improvement of motor development and postural control following intervention in children with sensorineural hearing loss and vestibular impairment. Int J Pediatr Otorhinolaryngol 68(9) (2004), 1141–1148. [DOI] [PubMed] [Google Scholar]
- [52].Rine RM, Roberts D, Corbin BA, McKean-Cowdin R, Varma R, Beaumont J, Slotkin J, Schubert MC New portable tool to screen vestibular and visual function--National Institutes of Health Toolbox initiative. J Rehabil Res Dev 49 (2012),209–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Rine RM, Schubert MC, Whitney SL, Roberts D, Redfern MS, Musolino MC, Roche JL, Steed DP, Corbin B, Marchetti GF, Beaumont J, Carey JP, Shepard NP, Jacobson GP, Wrisley DM, Hoffman HJ, Furman G, and Slotkin J. Vestibular function assessment using the NIH Toolbox. Neurology 80 (2013), S25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Snashall SE. Vestibular function tests in children. J Royal Society of Med 76 (1983), 555–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Spahr AJ, Dorman MF, Litvak LM, Van Wie S, Gifford RH, Loizou PC, Loiselle LM, Oakes T, and Cook S. Development and validation of the AzBio sentence lists. Ear Hear 33(1) (2012), 112–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Spahr AJ, Dorman MF, Litvak LM, Cook SJ, Loiselle LM, DeJong MD, Hedley-Williams A, Sunderhaus LS, Hayes CA, and Gifford RH. Development and validation of the Pediatric AzBio sentence lists. Ear Hear 35(4) (2014), 418–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Tomaz A, Gananca MM, Garcia AP, Kessler N, and Caovilla HH. Postural control in underachieving students. Braz J Otorhinolaryngol 80(2) (2014), 105–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Wechsler D, and Zhou X. Wechsler Abbreviated Scale of Intelligence (2nd ed.). NCS Pearson, Bloomington, MN, 2011. [Google Scholar]
- [59].Wiener-Vacher SR, Hamilton DA, Wiener SI. Vestibular activity and cognitive development in children: Perspectives. Front Integr Neurosci 92 (2013), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Xie Y, Bigelow RT, Frankenthaler SF, Studenski SA, Moffat SD, and Agrawal Y. Vestibular Loss in Older Adults Is Associated with Impaired Spatial Navigation: Data from the Triangle Completion Task. Front Neuro, 8, (2017) 173. [DOI] [PMC free article] [PubMed] [Google Scholar]


