Abstract
Background
Polymyxin B is the first-line therapy for Carbapenem-resistant organism (CRO) nosocomial pneumonia. However, clinical data for its pharmacokinetic/pharmacodynamic (PK/PD) relationship are limited. This study aimed to investigate the relationship between polymyxin B exposure and efficacy for the treatment of CRO pneumonia in critically ill patients, and to optimize the individual dosing regimens.
Methods
Patients treated with polymyxin B for CRO pneumonia were enrolled. Blood samples were assayed using a validated high-performance liquid chromatography-tandem mass spectrometry method. Population PK analysis and Monte Carlo simulation were performed using Phoenix NLME software. Logistic regression analyses and receiver operating characteristic (ROC) curve were employed to identify the significant predictors and PK/PD indices of polymyxin B efficacy.
Results
A total of 105 patients were included, and the population PK model was developed based on 295 plasma concentrations. AUCss,24 h/MIC (AOR = 0.97, 95% CI 0.95–0.99, p = 0.009), daily dose (AOR = 0.98, 95% CI 0.97–0.99, p = 0.028), and combination of inhaled polymyxin B (AOR = 0.32, 95% CI 0.11–0.94, p = 0.039) were independent risk factors for polymyxin B efficacy. ROC curve showed that AUCss,24 h/MIC is the most predictive PK/PD index of polymyxin B for the treatment of nosocomial pneumonia caused by CRO, and the optimal cutoff point value was 66.9 in patients receiving combination therapy with another antimicrobial. Model-based simulation suggests that the maintaining daily dose of 75 and 100 mg Q12 h could achieve ≥ 90% PTA of this clinical target at MIC values ≤ 0.5 and 1 mg/L, respectively. For patients unable to achieve the target concentration by intravenous administration, adjunctive inhalation of polymyxin B would be beneficial.
Conclusions
For CRO pneumonia, daily dose of 75 and 100 mg Q12 h was recommended for clinical efficacy. Inhalation of polymyxin B is beneficial for patients who cannot achieve the target concentration by intravenous administration.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13054-023-04448-z.
Keywords: Polymyxin B, Carbapenem-resistant organism, Nosocomial pneumonia, Dosing optimization, Pharmacokinetic/pharmacodynamic
Background
Over the last decade, nosocomial pneumonia caused by carbapenem-resistant organism (CRO) infection has become a significant important cause of mortality and morbidity worldwide, especially in critically ill patients [1, 2]. Due to the broad antimicrobial resistance among CRO, there is limited treatment option, and make it an extreme challenge [3, 4].
Polymyxins (polymyxin B and colistin), which were withdrawn from the market due to the high risk of nephrotoxicity and neurotoxicity in the 1970s, have been reused for their high sensitivity against CRO [5, 6]. And because of its more predictable pharmacokinetics and rapid antimicrobial activity, polymyxin B has become a preferred choice over colistin [7, 8]. Unfortunately, due to the early development and a subsequent lack of use in a clinical setting, there is little information about the pharmacokinetic/pharmacodynamic (PK/PD) relationship of polymyxin B against CRO pneumonia, and the optimal dosing remains controversial [9–11].
The latest guidelines recommend an area under the concentration-time curve across 24 h at steady state (AUCss,24 h) of 50–100 mg·h/L for polymyxins to achieve bactericidal activity against an isolate with a MIC of 2 mg/L (the EUCAST and CLSI breakpoints) [7]. However, this PK/PD target was mainly based on the results of limited in vitro and murine thigh infection models, and most evaluated colistin [9, 12]. Although Yang et al. recently confirmed that AUCss,24 h threshold of 50–100 mg·h/L was a good predictor of polymyxin B clinical response and acute kidney injury (AKI) risk in a retrospective study, it has to be pointed out that this study included patients with different types of infections, and was not focus on pneumonia [13]. It is well known that the PK/PD indices and targets of antibiotics are diversity among different types of infections [14, 15]. Moreover, according to the PK/PD analysis of murine lung infection model, the present recommended target is very likely to be suboptimal for the systemic treatment of pneumonia [16]. Therefore, it is necessary to re-evaluate whether this relationship applies to patients with CRO nosocomial pneumonia in prospective clinical trials.
At present, weight-based dosing regimen (1.25–1.5 mg/kg every 12 h) is recommended for polymyxin B [17, 18]. However, polymyxin B concentration varies widely in critically ill patients with this regimen, and almost 30% patients cannot achieve AUCss,24 h values within the target therapeutic window [19]. Moreover, Miglis et al. found that weight-based dosing strategies might be associated with increased toxicity in higher weight patients as well as insufficient concentration in lower weight patients [20]. Due to these inconsistent results, further research is needed to improve the characterization of polymyxin B PK, in order to identify the optimization of dose regimens.
The primary objective of this study was to investigate the relationship between polymyxin B exposure and efficacy in the treatment of CRO pneumonia and to determine the appropriate PK/PD target for this infection. In addition, Monte Carlo simulations were performed to select the optimal dosage regimens.
Methods
Study design and patients
This prospective study was conducted at two intensive care units (ICU) between January 2020 and December 2021 in the Second Xiangya Hospital of Central South University (Changsha, China). Patients were included if (a) age ≥ 18 years; (b) diagnosed with nosocomial pneumonia that developed more than 48 h after admission; (c) at least two consecutive samples on different days (time interval at least 24 h) showed the presence of CRO from bronchial secretions or bronchoalveolar lavage samples; (d) received intravenous polymyxin B treatment over 3 days. The exclusion criteria were as follows: (a) concomitant lung cancer with obstructive pneumonitis or cystic fibrosis; (b) solid organ transplantation; (c) hematologic malignancies and hematopoietic cell transplant recipients; (d) receiving renal replacement therapy. HAP was defined according to the 2016 clinical practice guidelines of the Infectious Diseases Society of America and the American Thoracic Society [21]. Determination of carbapenem susceptibility of CRO was followed by the European Committee on Antimicrobial Susceptibility Testing (EUCAST). Updated EUCAST Clinical Breakpoints of polymyxin B were sensitivity (S ≤ 2 mg/L) and drug resistance (R > 2 mg/L). The antimicrobial susceptibility testing was performed using the VITEK-2 Compact system with VITEK cards (0.5–16 mg/L for colistin) (bioMérieux, France). The following information was extracted from the electronic medical records: demographic and co-morbidity profiles, clinical and microbiological features of the infections, and the antimicrobial treatment regimens. Creatinine clearance (CrCL) was calculated using the Cockcroft–Gault equation. The endpoint was clinical efficacy and 30-day all-cause mortality. Assessment of clinical efficacy was conducted at the end of treatment, and 30-day mortality was recorded from the start of polymyxin B treatment. This prospective study was approved by the Ethics Committee of the Second Xiangya Hospital, Central South University. Informed consent was obtained from all patients or legal representatives of the patients (No. ChiCTR1900022231).
Drug administration and concentration determination
Polymyxin B was given to all patients empirically as a loading dose of 100–200 mg followed by a maintenance dose of 40–100 mg every 12 h for at least 3 days. The infusion time was at least 1 h. Aerosol delivery of polymyxin B (25 mg or 50 mg twice daily) was using a vibrating mesh nebulizer, synchronized with the inspiratory cycle of the ventilator. Two to six blood samples (2 mL) were randomly collected immediately before the seventh dose of polymyxin B and at 0, 1, 2, 4, 6, 8 and 10 h after the end of infusion. The supernatant was immediately stored at − 80 °C until analysis.
An established high-performance liquid chromatography-tandem mass spectrometry (HPLC–MS/MS) was used to measure the concentrations of polymyxin B1 and polymyxin B2 as described previously by the authors’ laboratory (The total concentration of polymyxin B = [polymyxin B1 concentration/polymyxin B1 molecular + polymyxin B2 concentration/polymyxin B2 molecular]*total polymyxin B molecular) [22]. The interday precision was < 12%, the intraday precision was < 9%, and the accuracy ranged from 96.1 to 110.4%. The limit of quantification (LLOD) was 0.03 mg/L, and all of the polymyxin B concentrations detected were over LLOD.
Population PK model and calculation of PK/PD indices
The Phoenix NLME program (version 8.1. Pharsight, A Certara Company, USA) with the method of first-order conditional estimation-extended least square method (FOCE-ELS) was used to develop the population PK model by analyzing polymyxin B concentration. The objective function value (OFV), goodness-of-fit plots and the reasonable of population PK parameters were used to selection of the structure model. The stepwise covariate modeling (SCM) approach was used to test the covariate model in this analysis; age, sex, body weight, alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TBIL), direct bilirubin (DBIL), serum albumin (ALB) and CrCL were evaluated as the covariates. The SCM consists of a forward selection step (the criterion is p < 0.05 for ΔOFV decreased ≥ 3.84) and a backward elimination step (the criterion is p < 0.001 for ΔOFV increased ≥ 10.83). In addition, the goodness-of-fit plots were used to assess the validity of the population PK model, the prediction-corrected visual predictive check (pcVPC) was used to assess the predictive performance of the key models. The bootstrap method was used to assess the accuracy.
The PK/PD indices included AUCss,24 h, AUCss,24 h/MIC, the peak and trough concentration at steady state (Cmax,ss and Cmin,ss), Cmax,ss /MIC and Cmin,ss /MIC. AUCss,24 h, Cmax,ss and Cmin,ss were calculated based on the empirical Bayesian (EBEs). For those patients with more than one baseline pathogen, AUCss,24 h/MIC, Cmax,ss /MIC and Cmin,ss /MIC evaluations were based on the pathogen with the highest MIC value.
Pharmacokinetic/Pharmacodynamic analysis for clinical efficacy and mortality
Clinical outcomes were classified as clinical success (CS) and clinical failure (CF), and were assessed by two physicians. CS was defined as a composite of survival; hemodynamic stability; body temperature < 38 °C, improved biochemistry indicators of infection; stable or improved PaO2/FiO2 ratio. Additionally, for patients with bacteremia, microbiological cure (no growth of the initial isolate in blood cultures) must be achieved by the end of the treatment [23–25]. Patients who did not meet all above criteria were classified as CF. 30-day all-cause mortality was recorded from the start of polymyxin B treatment.
Variables potentially related to clinical efficacy and 30-day all-cause mortality were assessed, including: demographics, co-morbidities, clinical conditions, dosage regimen and concentration of polymyxin B. To develop receiver operating characteristic (ROC) curves, the PK/PD indices AUCss,24 h/MIC, Cmax,ss/MIC and Cmin,ss/MIC were used as predictors of clinical efficacy. The area under the diagnostic curve (AUCROC) was calculated to evaluate the correlation of the above parameters with clinical efficacy and 30-day all-cause mortality. Youden index of the ROC curves was calculated by "sensitivity + specificity-1", and the values corresponding to the maximum Youden index is the optimal cutoff point value of the PK/PD indices.
Monte Carlo simulations of dosage regimens
Based on the final population PK models, the plasma concentration-time profile of 1,000 individuals was simulated. The dosages were selected according to the most commonly used regimens in clinical practice. The regimens were 100–200 mg loading dose followed by 75–150 mg every 12 h. The infusion time was set to 2 h.
Statistical analysis
Statistical analysis was performed with SPSS 24.0 (SPSS, IBM Company, Chicago, IL, USA) software. Continuous variables are presented as the mean ± standard deviation (SD) if normally distributed and were compared using Student’s t tests. The median and interquartile range (IQR) are presented for abnormally distributed data, and the Mann–Whitney U test was used. Categorical variables are expressed as counts and percentages, and the chi-square test or Fisher’s exact test was used. Spearman’s rank correlation coefficient (r) was used to analyze the correlation between Cmin,ss, Cmax,ss and AUCss,24 h. Univariate analysis was performed for all variables to identify possible predictors for clinical efficacy. Variables with a p < 0.05 were entered into the multivariate logistic regression models. A forward stepwise (likelihood ratio) method was performed to determine the predictors using a significance level of 0.05 for entry and 0.10 for removal from the model. 30-day all-cause mortality was evaluated with Cox regression model. P < 0.05 was considered statistically significant.
Results
Patients characteristics
During the study period, 132 patients (≥ 18 years) received polymyxin B therapy. Among them, 11 patients were non-HAP, five patients had no pathogenic microorganism result, four patients were solid transplant recipients, four patients received renal replacement and three patients received polymyxin B treatment ≤ 3 days. Thus, 105 patients were eventually enrolled. The demographic characteristics of all patients are summarized in Table 1. The median APACHE II score of these patients was 18 (IQR: 12, 25), and the rate of sepsis was 39.0% (41/105). The most common pathogenic bacteria were Acinetobacter baumannii (N = 86; 81.9%), followed by Klebsiella pneumoniae (N = 42; 40.0%) and Pseudomonas aeruginosa (N = 14; 13.3%). For most of our CRO stains, MIC values of polymyxin B were 1 mg/L, and ≤ 0.5 mg/L for three isolates of Klebsiella pneumoniae. MIC50 and MIC90 of polymyxin B were 1 mg/L for Acinetobacter baumannii, Klebsiella pneumoniae and Pseudomonas aeruginosa. The loading dose of polymyxin B was 100 mg (IQR: 100,150 mg). The median polymyxin B daily dose was 2.3 mg/kg (IQR: 2.0, 2.9 mg/kg) with a duration of 12 days (IQR: 9, 16).
Table 1.
Demographic data for 105 patients in PPK model
| Variable | Valuesa |
|---|---|
| Demographics | |
| Age (years) | 65 (IQR: 55, 76) |
| Gender (male) | 76 (72.4%) |
| Weight (Kg) | 55.0 (IQR:50.0, 65.0) |
| Clinical condition | |
| Albumin (g/L) | 30.7 (IQR: 27.9, 34.2) |
| Baseline creatinine clearance (mL/min) | 72.2 (IQR: 50.5, 111.5) |
| Baseline BUN (mmol/L) | 9.8 (IQR: 6.6, 15.8) |
| Mechanical ventilation | 81 (77.1%) |
| APACHEII scores | 18 (IQR: 12, 25) |
| Mortality rates | 28 (26.7%) |
| Comorbidities | |
| Sepsis | 41 (39.0%) |
| Pulmonary diseases | 15 (14.3%) |
| Heart disease | 58 (55.2%) |
| Diabetes mellitus | 19 (18.1%) |
| Chronic liver disease | 29 (27.6%) |
| Chronic renal dysfunction | 25 (23.8%) |
| Trauma | 5 (4.8%) |
| Solid tumor | 15 (14.3%) |
| Pathogens | |
| CRAB | 86 (81.9%) |
| CRKP | 42 (40.0%) |
| CRPA | 23 (21.9%) |
| PMB treatment | |
| PMB loading dose (mg) (n = 87) | 100 (IQR: 100, 150) |
| PMB loading dose (mg/kg) (n = 87) | 2.1 (IQR: 1.9, 2.3) |
| PMB daily dose (mg) | 150 (IQR: 100, 150) |
| PMB daily dose by weight (mg/kg/day) | 2.3 (IQR: 2.0, 2.9) |
| PMB treatment duration (days) | 12 (IQR: 9, 16) |
| PMB total dose (mg) | 1500 (IQR: 1100, 2175) |
| PMB total dose by weight (mg/kg/day) | 26.0 (IQR: 18.7, 38.2) |
| Combinational therapy | |
| Carbepenem | 36 (34.3%) |
| Tigecycline | 33 (31.4%) |
| Other β-lactam antibioticsb | 32 (30.5%) |
| Ceftazidime avibactam | 4 (3.8%) |
| Quinolone | 4 (3.8%) |
IQR Interquartile range, APACHE Acute physiology and chronic health evaluation, BUN Blood urea nitrogen. PMB Polymyxin B, CRAB Carbapenem-resistant acinetobacter baumannii, CRKP Carbapenem-resistant klebsiella pneumonia, CRPA Carbapenem-resistant pseudomonas aeruginosa
aCategorical data are number (%) of subjects, continuous data are expressed as median (interquartile range, IQR)
bother β-lactam antibiotics include cefoperazone/sulbactam (n = 23) and piperacillin/tazobactam (n = 9)
Population PK Model and polymyxin B exposure
The population PK model was developed based on 295 plasma concentrations obtained from 105 patients, each patient on average contributed three clinical samples. A two-compartment model fully described the data, and no covariate was statistically significant to PK parameters. A proportional error model was used to evaluate the residual variability. In addition, the shrinkage of the clearance between central compartment and peripheral compartment (CLd) and volume in peripheral compartment (Vp) were more than 50%, so the inter-individual variability (IIV) of CLd and Vp were fixed 0. Due to the highly correlation between the clearance in central compartment (CL) and the volume distribution in central compartment (Vc), the omega block was applied to improve the accuracy of IIV in each patient, and the estimated covariance between CL and Vc is 100%.
The goodness-of-fit plots in the final model are shown in Fig. 1. The plots were shown that the structure of the final model was not biased and that the model was acceptable. The result of pcVPC is presented in Fig. 2. Most concentrations were within the 90% CIs, indicating that the final model had a good description of the original data. The bootstrap results are shown in Table 2, which indicating qualified precision for the final population PK models. In the final model, the typical values of CL, Vc, CLd, and Vp were 1.56 L/h, 12.5 L, 2.41 L/h, and 29.9 L, respectively. The final PK model equations were as follows: , , , . The results of final PPK model are shown in Table 2.
Fig. 1.
Goodness-of-fit plots for the final population pharmacokinetic model. A Polymyxin B concentration versus population predicted concentrations (PRED); B Polymyxin B concentration versus individual predicted concentrations (IPRED); C Conditional weighted residuals versus population predicted concentrations (CWRES vs. PRED); D Conditional weighted residuals versus time (CWRES vs. Time); The blue lines in panels (C, D) represent smoothed regression lines
Fig. 2.

The prediction corrected-visual predictive check (pc-VPC) of the final population PK model. The red and black lines represent the 5th, 50th and 95th quantiles of the observed and predicted concentration, and the shaded area represents the simulation-based 90% confidence intervals
Table 2.
Population PK parameter estimates in the final model and bootstrap
| Final model | Bootstrap method | ||||
|---|---|---|---|---|---|
| Parameters (Unite) |
Estimate | Standard Error (%SE) |
Parameters (Unite) |
Estimate | Standard Error (%SE) |
| Fixed effect | Fixed effect | ||||
| CL (L/h) | 1.56 | 4.50 | CL (L/h) | 1.56 | 4.47 |
| CLd (L/h) | 2.41 | 11.8 | CLd (L/h) | 2.41 | 11.6 |
| Vc (L) | 12.5 | 5.67 | Vc (L) | 12.5 | 5.65 |
| Vp (L) | 29.9 | 17.3 | Vp (L) | 29.9 | 16.7 |
| Random effect (%) | Random effect (%) | ||||
| ωCL*Vc | 100 | 12.7 | ωCL*Vc | 100 | 12.5 |
| ωCL | 39.4 | 6.4 | ωCL | 39.4 | 6.16 |
| ωVc | 28.6 | 15.5 | ωVc | 28.6 | 15.3 |
| Residual error (%) | Residual error (%) | ||||
| Proportional residual (%) | 27.86 | 9.11 | Proportional residual (%) | 27.86 | 9.09 |
CL The clearance in central compartment, CLd The clearance between central compartment and peripheral compartment, Vc The volume distribution in central compartment, Vp The volume in peripheral compartment, ωCL*Vc The correlation of CL and Vc
The median values of Cmin,ss, Cmax,ss and AUCss,24 h were 1.9 mg/L (IQR:1.4, 2.7 mg/L), 5.9 mg/L (4.6, 7.1 mg/L) and 67.2 mg·h/L (IQR:54.8, 84.2 mg·h/L), respectively. Spearman’s rank correlation analysis showed that AUCss,24 h were positively correlated Cmin,ss, Cmax,ss (Additional file 1: Fig. S1a, Additional file 2: Fig. S1b).
Pharmacokinetic/Pharmacodynamic analysis for clinical efficacy and mortality
Among the 105 patients, the clinical success rate was 66.7% (70/105). Patients in the clinical success group were more likely to combine with inhaled polymyxin B (40.0% VS 17.1%, p = 0.012) than patients in the clinical failure group. The dosage (daily, total), duration and plasma concentrations (Cmin,ss, Cmax,ss, AUCss,24 h, Cmin,ss/MIC, Cmax,ss/MIC and AUCss,24 h/MIC) of polymyxin B were significantly higher (p < 0.05) in the clinical success group than in the clinical failure group (Table 3). Based on these results, a total of 12 possible risk factors were identified by the univariate analysis. These 12 variables (p < 0.05) were used to develop a multivariate logistic regression model. Finally, AUCss,24 h/MIC (AOR = 0.97, 95% CI 0.95–0.99, p = 0.009), daily dose (AOR = 0.98, 95% CI 0.97–0.99, p = 0.028), and combination of inhaled polymyxin B (AOR = 0.32, 95% CI 0.11–0.94, p = 0.039) were included in the final model. A ROC curve was used to calculate the discriminatory power of the model (AUC = 0.795) (Fig. 3). In addition, considering that AUCss,24 h and dose are directly correlated, the relationship between efficacy and dose normalized-AUCss,24 h/MIC was tested using the Covariate-Adjusted Residuals method to remove this intrinsic correlation (AOR = 0.62, 95% CI 0.38–1.01, p = 0.056).
Table 3.
Univariate and Multivariable logistic regression model for clinical efficacy
| Variables | CSa (n = 70) | CFa (n = 35) | pb | Adjusted OR (95% CI) | pc |
|---|---|---|---|---|---|
| Demographic parameters | |||||
| Female | 19 (27.1%) | 13 (37.1%) | 0.443 | ||
| Age (years) | 65.4 (56.0, 73.0) | 62.2 (51.0, 79.0) | 0.259 | ||
| Weight (Kg) | 59.5 (50.0, 65.0) | 54.0 (50.0, 60.0) | 0.109 | ||
| Comorbidities | |||||
| Sepsis | 22 (31.4%) | 19 (54.3%) | 0.060 | ||
| Pulmonary diseases | 12 (17.1%) | 3 (8.6%) | 0.105 | ||
| Heart disease | 40 (57.1%) | 18 (51.4%) | 0.332 | ||
| Diabetes mellitus | 12 (17.1%) | 7 (20.0%) | 0.868 | ||
| Chronic liver disease | 17 (24.3%) | 12 (34.3%) | 0.415 | ||
| Chronic renal dysfunction | 13 (18.6%) | 12 (34.3%) | 0.130 | ||
| Solid tumor | 10 (14.3%) | 5 (14.3%) | 0.871 | ||
| Clinical conditions | |||||
| Baseline CrCL (mL/min) | 90.3 (57.5, 102.1) | 73.0 (29.3, 104.9) | 0.092 | ||
| Albumin (g/L) | 30.6 (27.9, 32.4) | 32.0 (27.0, 35.9) | 0.326 | ||
| Baseline BUN (mmol/L) | 11.7 (6.2, 12.0) | 14.0 (7.3,18.2) | 0.246 | ||
| APACHEII score | 17.5 (12, 19) | 20.2 (12,25) | 0.091 | ||
| Mechanical ventilation | 49 (62.8%) | 32 (91.4%) | 0.109 | ||
| Pathogens and susceptibility | |||||
| CRAB | 56 (80.0%) | 30 (85.7%) | 0.897 | ||
| CRKP | 27 (38.6%) | 15 (42.9%) | 0.614 | ||
| CRPA | 13 (18.6%) | 10 (28.6%) | 0.52 | ||
| ≤ 0.5 mg/L | 3 (4.3%) | 0 (0.0%) | 0.999 | ||
| 1 mg/L | 67 (95.7%) | 35 (100%) | 0.999 | ||
| PMB treatment | |||||
| Daily dose (mg) | 144.7 (100.0, 150.0) | 117.0 (100.0,150.0) | < 0.0001 | 0.98 (0.97–0.99) | 0.028 |
| Daily dose/weight (mg/Kg) | 2.5 (2.1, 2.9) | 2.0 (1.8, 2.5) | 0.004 | ||
| Duration (day) | 13 (9, 16) | 11 (6, 14) | 0.034 | ||
| Total dosage (mg) | 1915.4 (1200.0, 2250.0) | 1284.0 (800.0, 1500.0) | 0.001 | ||
| Total dosage (mg/Kg) | 33.3 (22.5, 40.0) | 24.0 (15.4, 28.0) | 0.002 | ||
| Combined with inhaled PMB | 28 (40.0%) | 6 (17.1%) | 0.012 | 0.32 (0.11–0.94) | 0.039 |
| PMB concentration | |||||
| AUCss, 24 h (mg·h/L) | 78.6 (62.4, 84.2) | 60.6 (43.5, 66.6) | 0.002 | ||
| AUCss, 24 h /MIC | 80.5 (62.6, 86.9) | 61.9 (43.2, 68.7) |
0.001 0.056# |
0.97 (0.95–0.99) | 0.009 |
| Cmin,ss (μg/mL) | 2.4 (1.6, 2.8) | 1.8 (1.0, 2.5) | 0.015 | ||
| Cmin,ss /MIC | 2.4 (1.4, 3.1) | 1.7 (0.9, 1.9) | 0.011 | ||
| Cmax,ss,(μg/mL) | 6.8 (5.0, 7.6) | 5.1 (3.7, 6.9) | 0.005 | ||
| Cmax,ss /MIC | 6.7 (4.9, 7.6) | 5.0 (3.8, 6.8) | 0.003 | ||
| Combination therapy | |||||
| Carbapenems | 20 (28.6%) | 16 (45.7%) | 0.156 | ||
| Tigecycline | 21 (30.0%) | 12 (34.3%) | 0.865 | ||
| Ceftazidime avibactam | 3 (4.3%) | 1 (2.9%) | 0.667 | ||
| Other β-lactamd | 23 (32.8%) | 9 (25.7%) | 0.321 | ||
| Quinolone | 4 (5.7%) | 0 (0.0%) | 0.999 | ||
CI Confidence interval, OR Odds ratio, CrCL Creatinine clearance, APACHE Acute physiology and chronic health evaluation, BUN Blood urea nitrogen, PMB Polymyxin B, CRAB Carbapenem-resistant acinetobacter baumannii, CRKP Carbapenem-resistant klebsiella pneumonia, CRPA Carbapenem-resistant pseudomonas aeruginosa, AUCss, 24 h The area under the plasma concentration–time curve across 24 h at steady state, Cmin,ss Steady-state trough plasma concentration, Cmax,ss Steady-state peak plasma concentration, CS Clinical success, CF Clinical failure
aCategorical data are number (%) of subjects, continuous data are expressed as median (interquartile range, IQR)
bderived from univariate analysis
cderived from Cox regression analysis
dother β-lactam antibiotics include cefoperazone/sulbactam (n = 23) and piperacillin/tazobactam (n = 9)
#adjusted by daily dose. Bold indicates data with significant differences (p < 0.05)
Fig. 3.
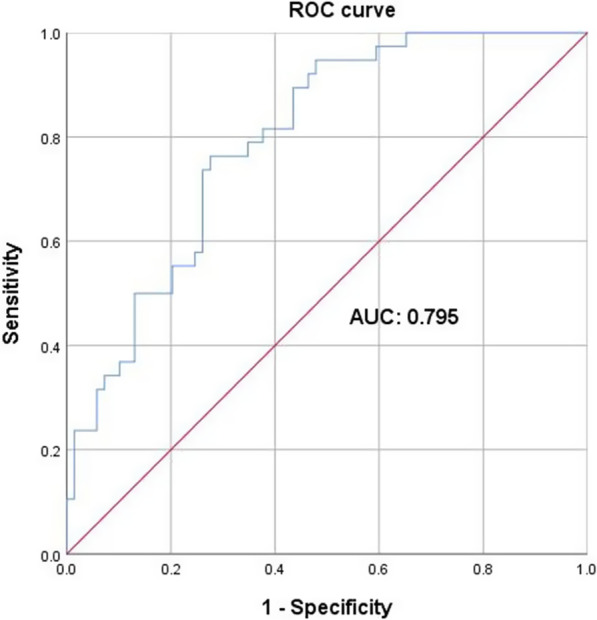
ROC of the final multivariate logistic regression model
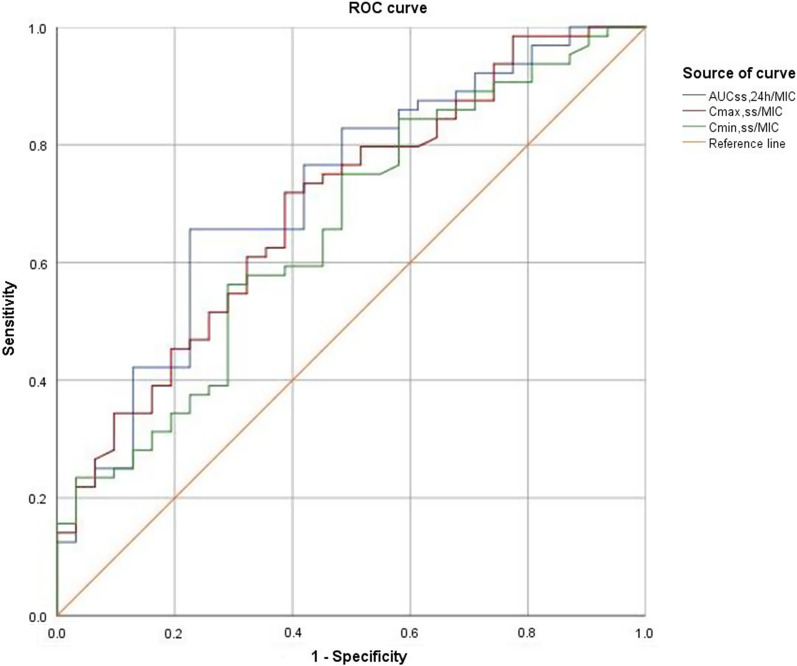
In addition, the ROC curves support that the AUCss,24 h/MIC (AUCROC = 0.719) was superior to the other two PK/PD indices for the prediction of clinical efficacy. When the Youden index was the largest (1.401), the corresponding optimal cutoff point value of AUCss,24 h/MIC was 66.9. The predictive sensitivity and the specificity of this value were 65.6% and 77.4%, respectively (Fig. 4).
Fig. 4.
The area under the ROC curve of polymyxin B PK/PD indices in prediction of clinical efficacy
Subgroup analysis showed that combination of inhaled polymyxin B was a significant predictor for the clinical efficacy (OR: 0.21, 95% CI: 0.06–0.79, p = 0.022) of the 51 patients with AUCss,24 h/MIC < 66.9. The clinical success rate was significantly higher in the intravenous (IV) plus inhaled (IH) group (71.4% vs. 35.1%, p = 0.017). However, of the 54 patients with AUCss,24 h/MIC ≥ 66.9, the combination of inhaled polymyxin B was not a significant predictor of the clinical efficacy (OR: 0.46, 95% CI: 0.09–2.46, p = 0.365), the clinical success rate in IV + IH group (90.0%) was higher than that in IV group (82.3%), but there was no difference between the two groups (p = 0.279) (Fig. 5).
Fig. 5.
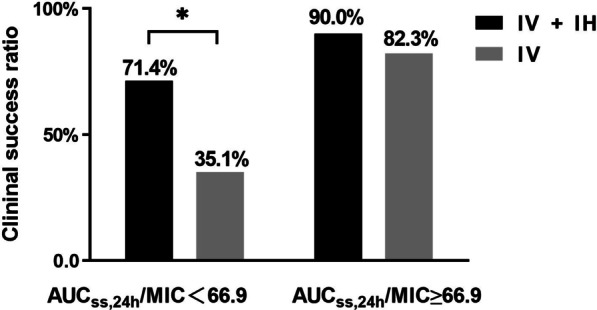
Comparison of clinical efficacy rates of polymyxin B in different subgroups. IV, intravenous polymyxin B; IH, inhaled polymyxin B; * represented p < 0.05
The 30-day all-cause mortality was 26.7% (28/105). None of the investigated PK/PD indices was significantly associated with 30-day mortality, and patients with higher APACHEII score, shorter treatment duration and clinical failure were associated with higher mortality in Cox regression model (Additional file 3: Table S1). ROC analysis was not conducted for mortality since no PK/PD index correlated with it was found in Cox regression model.
Monte Carlo simulations of dosage regimens
Our PK/PD analysis results showed that AUCss,24 h/MIC over 66.9 was associated with better clinical efficacy. Therefore, this cutoff value was adopted as PK/PD target and the probability of target attainment (PTA) was estimated for seven different MICs ranging from 0.125 to 8 mg/L on day 1 and day 4, respectively.
For an MIC less than 0.5 mg/L, the PTAs of all simulated regimens were greater than 90% on Day 4; When the MIC value was less than 1 mg/L, regimens from 100 to 150 mg every 12 h achieved the target exposure of AUCss,24 h/MIC over 66.9. None of the simulated regimens achieved adequate target attainment at the current CLSI and EUCAST breakpoint of 2 mg/L. Furthermore, we found that a loading dose made it possible to achieve the target PTA on Day 1, indicating that the loading dose is essential for polymyxin B treatments (Fig. 6).
Fig. 6.
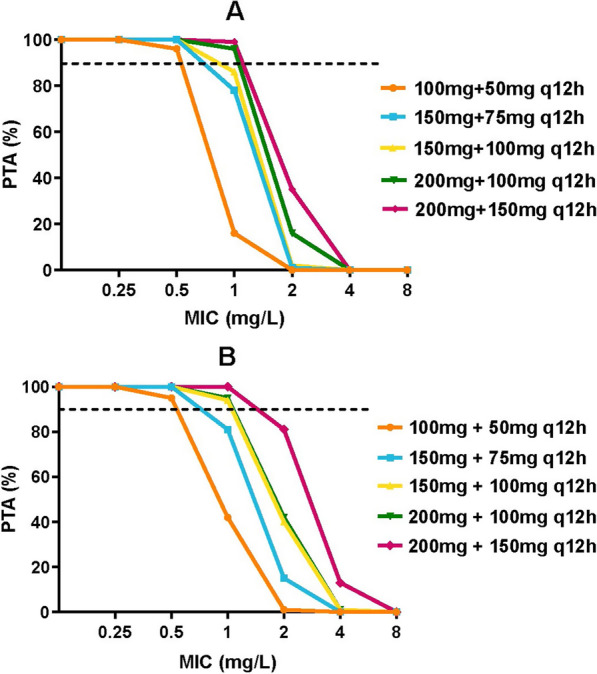
Probability of target attainment (PTA) for polymyxin B regimens on day 1 (A) and day 4 (B)
Discussion
This study investigated the exposure-response relationship of polymyxin B in the treatment of CRO pneumonia in critically ill patients, as well as explored the optimal dosage regimens for these patients. As a result, AUCss,24 h/MIC is the most predictive PK/PD index of polymyxin B against this infection, with a clinical cutoff value of 66.9. The daily dose of 75 mg and 100 mg Q12 h could achieve ≥ 90% PTA of this clinical target at MIC values ≤ 0.5 and 1 mg/L, respectively.
Several clinical studies have identified that the severity of disease and polymyxin B dosage were significant associating with its efficacy, in according with these results, we found that polymyxin B exposure at the site of infection (daily dose, AUCss,24 h/MIC and combined with inhalation) was the significant predictor of its clinical efficacy in the treatment of CRO pneumonia [26–28].
In vitro PD study identified the polymyxins against gram-negative bacteria in a rapid concentration-dependent way, which makes the fAUC/MIC and fCmax/MIC the reasonable PK/PD indices [29]. Previous murine thigh and lung infection models identified that fAUC/MIC was predictive for the PK/PD index of polymyxins against gram-negative bacteria [16]. Our real-world data further confirmed this finding that AUCss,24 h/MIC is the PK/PD parameter most closely linked to clinical outcomes. In addition, Cmax,ss/MIC also showed good correlation with polymyxin B efficacy (AUCROC = 0.696; p = 0.002), and we found that Cmax,ss was positively correlated with AUCss,24 h (Additional file 1: Fig. S1a, Additional file 2: Fig. S1b). In the clinical setting, since obtaining multiple samples throughout a dosing interval to estimate AUCss,24 h is not always feasible, the limited sampling strategies might be more applicable in clinical practice to assist therapeutic drug monitoring of polymyxin B, further investigation with a large sample may help to confirm this correlation.
According to the results of mouse lung infection models, the present recommended PK/PD target (AUCss,24 h of 50–100 mg·h/L) is supposed to be suboptimal for the systemic treatment of pneumonia [7, 16]. However, using the ROC curve, we identified the clinical cutoff value of AUCss,24 h/MIC (66.9), as the MIC values of polymyxin B for most of the CRO strains in our study are 1 mg/L, it seems that polymyxin B can lead to favorable clinical outcomes in pneumonia patients with AUCss,24 h > 66.9 (85.2%). The causes of this inconsistency might be as follows: 1. previous preclinical studies investigated the PK/PD target in the neutropenic murine model, but none of our patients was immunodeficient, and they might response better to the antibiotic treatment; 2. the recommended PK/PD exposure targets were derived from studies involving polymyxins monotherapy, however, all of our patients received combination therapy which is advantageous in the polymyxins treatments. Therefore, the clinical cutoff value of AUCss,24 h/MIC of 66.9 found in our study might be a promising PK/PD target for polymyxin B efficacy in patients receiving combination therapy with another antimicrobial, further study with larger sample is needed to confirm the target.
At present, weight-based dosing regimen is recommended for polymyxin B [17]. However, the relationship between body weight and polymyxin B PK parameters remains controversial [17, 20, 22]. We found no correlation between body weight and polymyxin B PK parameters in this study, which might due to the limited samples of the PPK model and a relatively narrow distribution of patient weights (IQR 55–76 kg), future PPK researches with rich sampling schedules are needed to illuminate the pharmacokinetic characteristics of polymyxin B, and to identify the optimization of dose regimens. According to the results of Monte Carlo simulation, regimens from 100 to 150 mg Q12 h and 75 mg Q12 h could achieve the efficacious target at MIC values ≤ 1 and 0.5 mg/L, respectively. Considering that most of the polymyxin B MIC distributions and MIC50/MIC90 values for the clinical isolates CRO strains are between 0.5 and 1 mg/L, and polymyxin B daily dose over 200 mg is found to be significantly associated with AKI [26, 30, 31], therefore, a maintenance dose of 75 mg or 100 mg Q12 h might be appropriate.
Adjunctive polymyxin inhale therapy for MDR gram-negative HAP or ventilator associated pneumonia (VAP) is recommended by the guideline [7, 32–34], and in our study, it was also an independent predictor of the clinical efficacy of polymyxin B. Interestingly, additional use of aerosolized polymyxin B did not significantly improve the clinical efficacy in the high exposure (AUCss,24 h/MIC > 66.9) subgroup. However, in the low exposure (AUCss,24 h/MIC < 66.9) subgroup, the use of aerosolized polymyxin B was an independent factor associated with favorable clinical outcome. These results were consistent with the finding by Chen et al. [35], that low-dose intravenous plus inhaled polymyxin B can significantly improve the clinical efficacy of the treatment of VAP. These findings indicated that combining inhalation polymyxin B is especially important for patients with lower exposure. Moreover, with the widespread clinical application of polymyxin B, increased MIC value and resistance have been reported [36–38]. In order to achieve the PK/PD target for these less sensitive bacteria, higher intravenous dosage are required, which may exceed the AKI threshold. Accordingly, combination of inhaled polymyxin B may be a solution to balance the efficacy and toxicity. Therefore, for the treatment of CRO pneumonia, inhaled polymyxin B can not only improve the efficacy, but also avoid the occurrence of AKI.
This study has several limitations. First, the sample size was limited, thus the ability to evaluating the impact of covariates on the population PK parameters is restricted. Second, we did not assess the free concentration of polymyxin B, considering the large protein binding variation among patients [39], the total concentration of polymyxin B might not be in accordance with the unbound fraction, which is considered to be pharmacologically active. To better evaluate the PK/PD relationship of polymyxin B, further study using free drug concentration is needed. Third, polymyxin B MIC values were determined by VITEK 2 automated system in our study, which might lead to onefold to twofold bias of the MIC values, further research using more precise measurement such as broth microdilution (BMD) is needed to clarify our exposure-response results. Last, due to the limited sample size, we cannot compare the efficacy between different inhaled polymyxin B dosages, to further optimize the regimens for pneumonia, larger scale, multicenter prospective studies are needed.
Conclusions
In conclusion, this study investigated the PK/PD relationship and the optimal dosage regimens of polymyxin B against CRO pneumonia in critically ill patients, and we found that AUCss,24 h/MIC was the reliable predictive PK/PD index with the target of 66.9 for the treatment of nosocomial pneumonia caused by CRO in patients receiving combination therapy with another antimicrobial. Model-based simulation suggests 75 mg and 100 mg Q12 h maintenance dosage to achieve the comparable efficacy threshold. For patients unable to achieve the target concentration by intravenous administration, adjunctive inhalation of polymyxin B would be beneficial.
Supplementary Information
Additional file 1. Fig. S1a Spearman’s rank correlation between peak, trough concentrations and AUCss, 24 h (A) scatterplot of the peak plasma concentrations
Additional file 2. Fig. S1b Spearman’s rank correlation between peak, trough concentrations and AUCss, 24 h (B) scatterplot of the trough plasma concentrations. AUCss, 24 h, the area under the plasma concentration-time curve across 24 hours at steady state
Additional file 3. Table S1. Univariate and Cox regression analysis of 30-day mortality
Abbreviations
- CRO
Carbapenem-resistant organism
- HAP
Hospital acquired pneumonia
- VAP
Ventilator-associated pneumonia
- AKI
Acute kidney injury
- PK
Pharmacokinetics
- PD
Pharmacodynamics
- PPK
Population pharmacokinetics
- AUCss,24 h
Area under the concentration-time curve across 24 h at steady state
- MIC
Minimum inhibitory concentration
- Cmin,ss
Trough concentration at steady state
- Cmax,ss
Peak concentration at steady state
- CLSI
Clinical and laboratory standards institute
- EUCAST
European Committee on Antimicrobial Susceptibility Testing
- HAP
Hospital acquired pneumonia
- CrCL
Creatinine clearance
- CF
Clinical failure
- CS
Clinical success
- APACHE II
Acute Physiology and Chronic Health Enquiry
- LLOD
Limit of quantification
- FOCE-ELS
First-order conditional estimation-extended least square
- OFV
Objective function value
- SCM
Stepwise covariate modeling
- pcVPC
Prediction-corrected visual predictive check
- EBEs
Empirical Bayesian
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- TBIL
Total bilirubin
- DBIL
Direct bilirubin
- ALB
Serum albumin
- CLd
Central compartment and peripheral compartment
- Vp
Volume in peripheral compartment
- IIV
Inter-individual variability
- CL
Central compartment
- Vc
Volume distribution in central compartment
- IV
Intravenous
- IH
Inhaled
- PTA
Probability of target attainment
- SD
Standard deviation
- IQR
Interquartile range
- HR
Hazard ratio
- OR
Odds ratios
- AOR
Adjusted odds ratios
- ROC
Receiver operating characteristic
- AUCROC
Area under the diagnostic curve
Author contributions
TTT, YL and YGZ contributed to data acquisition, analysis and interpretation. YL and YGZ prepared the figures. TTT, YGZ drafted the manuscript and gave approval of the final version to be submitted. PX, MY, YJZ and WJM supervised the research and revised the manuscript. BKZ and DXX designed the research. All authors read and approved the final manuscript.
Funding
This work was supported by the Scientific Research Project of Hunan Provincial Health Commission (No. 202103040800) and the Natural Science Foundation of Hunan Province (No. 2021JJ30969).
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The current study was approved by the Ethics Committees of the Second Xiangya Hospital, Central South University (No. ChiCTR1900022231) and waived informed consent.
Consent for publication
All authors have consented to the publication of the present manuscript.
Competing interests
All authors report no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Tiantian Tang and Ying Li have contributed equally to this work
Bikui Zhang and Yangang Zhou share the last authorship
References
- 1.Gupta R, Malik A, Rizvi M, Ahmed M, Singh A. Epidemiology of multidrug-resistant Gram-negative pathogens isolated from ventilator-associated pneumonia in ICU patients. J Glob Antimicrob Resist. 2017;9:47–50. doi: 10.1016/j.jgar.2016.12.016. [DOI] [PubMed] [Google Scholar]
- 2.Huang Y, Jiao Y, Zhang J, Xu J, Cheng Q, Li Y, Liang S, Li H, Gong J, Zhu Y, et al. Microbial etiology and prognostic factors of ventilator-associated pneumonia: a multicenter retrospective study in Shanghai. Clin Infect Dis. 2018;67(suppl_2):S146–52. doi: 10.1093/cid/ciy686. [DOI] [PubMed] [Google Scholar]
- 3.Paul M, Carrara E, Retamar P, Tangden T, Bitterman R, Bonomo RA, de Waele J, Daikos GL, Akova M, Harbarth S, et al. European society of clinical microbiology and infectious diseases (ESCMID) guidelines for the treatment of infections caused by multidrug-resistant Gram-negative bacilli (endorsed by European society of intensive care medicine) Clin Microbiol Infect. 2022;28(4):521–547. doi: 10.1016/j.cmi.2021.11.025. [DOI] [PubMed] [Google Scholar]
- 4.Doi Y. Treatment options for Carbapenem-resistant Gram-negative bacterial infections. Clin Infect Dis. 2019;69(Suppl 7):S565–75. doi: 10.1093/cid/ciz830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Justo JA, Bosso JA. Adverse reactions associated with systemic polymyxin therapy. Pharmacotherapy. 2015;35(1):28–33. doi: 10.1002/phar.1493. [DOI] [PubMed] [Google Scholar]
- 6.Li J, Nation RL, Turnidge JD, Milne RW, Coulthard K, Rayner CR, Paterson DL. Colistin: the re-emerging antibiotic for multidrug-resistant Gram-negative bacterial infections. Lancet Infect Dis. 2006;6(9):589–601. doi: 10.1016/S1473-3099(06)70580-1. [DOI] [PubMed] [Google Scholar]
- 7.Tsuji BT, Pogue JM, Zavascki AP, Paul M, Daikos GL, Forrest A, Giacobbe DR, Viscoli C, Giamarellou H, Karaiskos I, et al. International consensus guidelines for the optimal use of the polymyxins: endorsed by the American college of clinical pharmacy (ACCP), European society of clinical microbiology and infectious diseases (ESCMID), Infectious diseases society of America (IDSA), International society for anti-infective pharmacology (ISAP), Society of critical care medicine (SCCM), and Society of infectious diseases pharmacists (SIDP) Pharmacotherapy. 2019;39(1):10–39. doi: 10.1002/phar.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang P, Zhang Q, Zhu Z, Feng M, Sun T, Yang J, Zhang X. Population pharmacokinetics and limited sampling strategy for therapeutic drug monitoring of polymyxin B in Chinese patients with multidrug-resistant gram-negative bacterial infections. Front Pharmacol. 2020;11:829. doi: 10.3389/fphar.2020.00829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bergen PJ, Bulitta JB, Forrest A, Tsuji BT, Li J, Nation RL. Pharmacokinetic/pharmacodynamic investigation of colistin against Pseudomonas aeruginosa using an in vitro model. Antimicrob Agents Chemother. 2010;54(9):3783–3789. doi: 10.1128/AAC.00903-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Onufrak NJ, Rao GG, Forrest A, Pogue JM, Scheetz MH, Nation RL, Li J, Kaye KS. Critical need for clarity in polymyxin B dosing. Antimicrob Agents Chemother. 2017;61(5):e00208–e217. doi: 10.1128/AAC.00208-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manchandani P, Thamlikitkul V, Dubrovskaya Y, Babic JT, Lye DC, Lee LS, Tam VH. Population pharmacokinetics of polymyxin B. Clin Pharmacol Ther. 2018;104(3):534–538. doi: 10.1002/cpt.981. [DOI] [PubMed] [Google Scholar]
- 12.Tran TB, Velkov T, Nation RL, Forrest A, Tsuji BT, Bergen PJ, Li J. Pharmacokinetics/pharmacodynamics of colistin and polymyxin B: are we there yet? Int J Antimicrob Agents. 2016;48(6):592–597. doi: 10.1016/j.ijantimicag.2016.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang J, Liu S, Lu J, Sun T, Wang P, Zhang X. An area under the concentration-time curve threshold as a predictor of efficacy and nephrotoxicity for individualizing polymyxin B dosing in patients with carbapenem-resistant gram-negative bacteria. Crit Care. 2022;26(1):320. doi: 10.1186/s13054-022-04195-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tangden T, Ramos Martin V, Felton TW, Nielsen EI, Marchand S, Bruggemann RJ, Bulitta JB, Bassetti M, Theuretzbacher U, Tsuji BT, et al. The role of infection models and PK/PD modelling for optimising care of critically ill patients with severe infections. Intensive Care Med. 2017;43(7):1021–1032. doi: 10.1007/s00134-017-4780-6. [DOI] [PubMed] [Google Scholar]
- 15.Zhou Y, Xu P, Li H, Wang F, Yan H, Liang W, Xiang D, Zhang B, Banh HL. Population pharmacokinetics and exposure-response analysis of tigecycline in patients with hospital-acquired pneumonia. Br J Clin Pharmacol. 2021;87(7):2838–2846. doi: 10.1111/bcp.14692. [DOI] [PubMed] [Google Scholar]
- 16.Dudhani RV, Turnidge JD, Nation RL, Li J. fAUC/MIC is the most predictive pharmacokinetic/pharmacodynamic index of colistin against Acinetobacter baumannii in murine thigh and lung infection models. J Antimicrob Chemother. 2010;65(9):1984–1990. doi: 10.1093/jac/dkq226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kubin CJ, Nelson BC, Miglis C, Scheetz MH, Rhodes NJ, Avedissian SN, Cremers S, Yin MT. Population pharmacokinetics of intravenous polymyxin B from clinical samples. Antimicrob Agents Chemother. 2018;62(3):e01493–e1517. doi: 10.1128/AAC.01493-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie J, Roberts JA, Lipman J, Cai Y, Wang H, Zhao N, Xu X, Yang S, Li Y, Zhang K. Pharmacokinetic/pharmacodynamic adequacy of polymyxin B against extensively drug-resistant Gram-negative bacteria in critically ill, general ward and cystic fibrosis patient populations. Int J Antimicrob Agents. 2020;55(6):105943. doi: 10.1016/j.ijantimicag.2020.105943. [DOI] [PubMed] [Google Scholar]
- 19.Hanafin PO, Nation RL, Scheetz MH, Zavascki AP, Sandri AM, Kwa AL, Cherng BPZ, Kubin CJ, Yin MT, Wang J, et al. Assessing the predictive performance of population pharmacokinetic models for intravenous polymyxin B in critically ill patients. CPT Pharmacomet Syst Pharmacol. 2021;10(12):1525–1537. doi: 10.1002/psp4.12720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miglis C, Rhodes NJ, Avedissian SN, Kubin CJ, Yin MT, Nelson BC, Pai MP, Scheetz MH. Population pharmacokinetics of polymyxin B in acutely Ill adult patients. Antimicrob Agents Chemother. 2018;62(3):e01475–e1517. doi: 10.1128/AAC.01475-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, Palmer LB, Napolitano LM, O'Grady NP, Bartlett JG, Carratala J, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the infectious diseases society of America and the American thoracic society. Clin Infect Dis. 2016;63(5):e61–111. doi: 10.1093/cid/ciw353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y, Deng Y, Zhu ZY, Liu YP, Xu P, Li X, Xie YL, Yao HC, Yang L, Zhang BK, et al. Population pharmacokinetics of polymyxin B and dosage optimization in renal transplant patients. Front Pharmacol. 2021;12:727170. doi: 10.3389/fphar.2021.727170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Furtado GH, d'Azevedo PA, Santos AF, Gales AC, Pignatari AC, Medeiros EA. Intravenous polymyxin B for the treatment of nosocomial pneumonia caused by multidrug-resistant Pseudomonas aeruginosa. Int J Antimicrob Agents. 2007;30(4):315–319. doi: 10.1016/j.ijantimicag.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 24.Paul M, Daikos GL, Durante-Mangoni E, Yahav D, Carmeli Y, Benattar YD, Skiada A, Andini R, Eliakim-Raz N, Nutman A, et al. Colistin alone versus colistin plus meropenem for treatment of severe infections caused by carbapenem-resistant Gram-negative bacteria: an open-label, randomised controlled trial. Lancet Infect Dis. 2018;18(4):391–400. doi: 10.1016/S1473-3099(18)30099-9. [DOI] [PubMed] [Google Scholar]
- 25.Laessig KA. End points in hospital-acquired pneumonia and/or ventilator-associated pneumonia clinical trials: food and drug administration perspective. Clin Infec Dis. 2010;51(Suppl 1):S117–S119. doi: 10.1086/653059. [DOI] [PubMed] [Google Scholar]
- 26.Elias LS, Konzen D, Krebs JM, Zavascki AP. The impact of polymyxin B dosage on in-hospital mortality of patients treated with this antibiotic. J Antimicrob Chemother. 2010;65(10):2231–2237. doi: 10.1093/jac/dkq285. [DOI] [PubMed] [Google Scholar]
- 27.Nelson BC, Eiras DP, Gomez-Simmonds A, Loo AS, Satlin MJ, Jenkins SG, Whittier S, Calfee DP, Furuya EY, Kubin CJ. Clinical outcomes associated with polymyxin B dose in patients with bloodstream infections due to carbapenem-resistant Gram-negative rods. Antimicrob Agents Chemother. 2015;59(11):7000–7006. doi: 10.1128/AAC.00844-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin YW, Aye SM, Rao G, Zhou QT, Chan HK, Li J. Treatment of infections caused by Gram-negative pathogens: current status on the pharmacokinetics/pharmacodynamics of parenteral and inhaled polymyxins in patients. Int J Antimicrob Agents. 2020;56(6):106199. doi: 10.1016/j.ijantimicag.2020.106199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheah SE, Wang J, Nguyen VT, Turnidge JD, Li J, Nation RL. New pharmacokinetic/pharmacodynamic studies of systemically administered colistin against Pseudomonas aeruginosa and Acinetobacter baumannii in mouse thigh and lung infection models: smaller response in lung infection. J Antimicrob Chemother. 2015;70(12):3291–3297. doi: 10.1093/jac/dkv267. [DOI] [PubMed] [Google Scholar]
- 30.Rigatto MH, Behle TF, Falci DR, Freitas T, Lopes NT, Nunes M, Costa LW, Zavascki AP. Risk factors for acute kidney injury (AKI) in patients treated with polymyxin B and influence of AKI on mortality: a multicentre prospective cohort study. J Antimicrob Chemother. 2015;70(5):1552–1557. doi: 10.1093/jac/dku561. [DOI] [PubMed] [Google Scholar]
- 31.John JF, Falci DR, Rigatto MH, Oliveira RD, Kremer TG, Zavascki AP. Severe infusion-related adverse events and renal failure in patients receiving high-dose intravenous polymyxin B. Antimicrob Agents Chemother. 2017;62(1):e01617–e1717. doi: 10.1128/AAC.01617-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sobieszczyk ME, Furuya EY, Hay CM, Pancholi P, Della-Latta P, Hammer SM, Kubin CJ. Combination therapy with polymyxin B for the treatment of multidrug-resistant Gram-negative respiratory tract infections. J Antimicrob Chemother. 2004;54(2):566–569. doi: 10.1093/jac/dkh369. [DOI] [PubMed] [Google Scholar]
- 33.Pereira GH, Muller PR, Levin AS. Salvage treatment of pneumonia and initial treatment of tracheobronchitis caused by multidrug-resistant Gram-negative bacilli with inhaled polymyxin B. Diagn Microbiol Infect Dis. 2007;58(2):235–240. doi: 10.1016/j.diagmicrobio.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 34.Lin YW, Zhou Q, Onufrak NJ, Wirth V, Chen K, Wang J, Forrest A, Chan HK, Li J. Aerosolized polymyxin B for treatment of respiratory tract infections: determination of pharmacokinetic-pharmacodynamic indices for aerosolized polymyxin B against pseudomonas aeruginosa in a mouse lung infection model. Antimicrob Agents Chemother. 2017;61(8):e00211–e217. doi: 10.1128/AAC.00211-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu J, Shao M, Xu Q, Liu F, Pan X, Wu J, Xiong L, Wu Y, Tian M, Yao J, et al. Low-dose intravenous plus inhaled versus intravenous polymyxin B for the treatment of extensive drug-resistant Gram-negative ventilator-associated pneumonia in the critical illnesses: a multi-center matched case-control study. Ann Intensive Care. 2022;12(1):72. doi: 10.1186/s13613-022-01033-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lima WG, Brito JCM, Cardoso BG, Cardoso VN, de Paiva MC, de Lima ME, Fernandes SOA. Rate of polymyxin resistance among Acinetobacter baumannii recovered from hospitalized patients: a systematic review and meta-analysis. Eur J Clin Microbiol Infect Dis. 2020;39(8):1427–1438. doi: 10.1007/s10096-020-03876-x. [DOI] [PubMed] [Google Scholar]
- 37.Kaye KS, Pogue JM, Tran TB, Nation RL, Li J. Agents of last resort: polymyxin resistance. Infect Dis Clin North Am. 2016;30(2):391–414. doi: 10.1016/j.idc.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 38.Capone A, Giannella M, Fortini D, Giordano A, Meledandri M, Ballardini M, Venditti M, Bordi E, Capozzi D, Balice MP, et al. High rate of colistin resistance among patients with carbapenem-resistant Klebsiella pneumoniae infection accounts for an excess of mortality. Clin Microbiol Infect. 2013;19(1):E23–30. doi: 10.1111/1469-0691.12070. [DOI] [PubMed] [Google Scholar]
- 39.Sandri AM, Landersdorfer CB, Jacob J, Boniatti MM, Dalarosa MG, Falci DR, Behle TF, Bordinhao RC, Wang J, Forrest A, et al. Population pharmacokinetics of intravenous polymyxin B in critically ill patients: implications for selection of dosage regimens. Clin Infect Dis. 2013;57(4):524–531. doi: 10.1093/cid/cit334. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Fig. S1a Spearman’s rank correlation between peak, trough concentrations and AUCss, 24 h (A) scatterplot of the peak plasma concentrations
Additional file 2. Fig. S1b Spearman’s rank correlation between peak, trough concentrations and AUCss, 24 h (B) scatterplot of the trough plasma concentrations. AUCss, 24 h, the area under the plasma concentration-time curve across 24 hours at steady state
Additional file 3. Table S1. Univariate and Cox regression analysis of 30-day mortality
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.