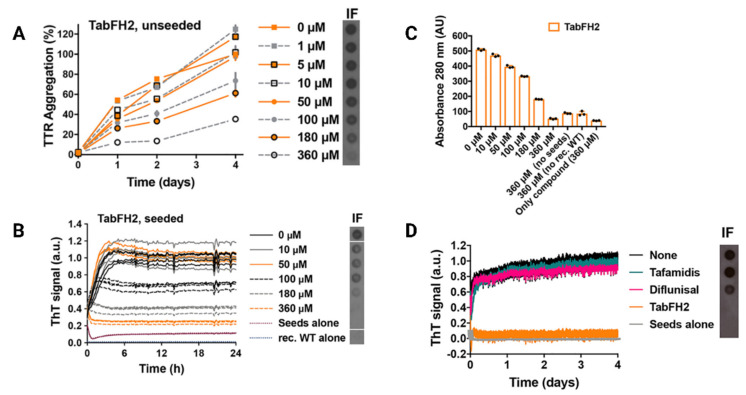
Figure 4.
TabFH2 inhibits TTR aggregation and amyloid seeding caused by ATTR ex vivo seeds. (A) Inhibition of TTR aggregation by TabFH2 in the absence of seeds, measured by absorbance at 400 nm. Increasing amounts of TabFH2 were added to 1 mg/mL of recombinant WT TTR, and the sample was incubated for 4 days at pH 4.3. Absorbance measured after 4 days of incubation in the absence of TabFH2 was considered 100% aggregation, because no soluble TTR was detected (n = 3). Error bars, S.D. Right inset, anti-TTR dot-blot of insoluble fractions (IF) collected by centrifugation after 4 days of incubation. (B) Inhibition of amyloid seeding by TabFH2 at pH 4.3, monitored by ThT fluorescence. Increasing amounts of TabFH2 were added to 0.5 mg/mL of recombinant WT TTR and 30 ng/L of ATTR-D38A seeds. All replicates are shown (n = 4). a.u., arbitrary units. Inset, anti-TTR dot-blot of IF collected by centrifugation after 24 h of incubation. All samples were spotted onto the same nitrocellulose membrane and subjected to the same procedure; splicing was needed for presentation purposes. (C) Protein content quantification of the insoluble fractions collected from B, measured by 280 nm absorbance. AU, absorbance units. The reduction in ThT fluorescence observed in B correlates with the decrease in total protein and TTR content in the IF, shown in the right inset of B. (D) Comparison of inhibition of amyloid seeding by tafamidis, diflunisal, and TabFH2 when incubated for 4 days, measured by ThT fluorescence. A 360 M inhibitor was added to 0.5 mg/mL of recombinant WT TTR and 30 ng/L of ATTR-D38A seeds (n = 3). Error bars, S.D. Inset, anti-TTR dot blot of IF collected by centrifugation after 4 days of incubation. Modified with permission from L. Saelices et al., 2019 [60].