Figure 4. Enhanced conformational heterogeneity stabilizes an inactive intracellular conformation of TM6.
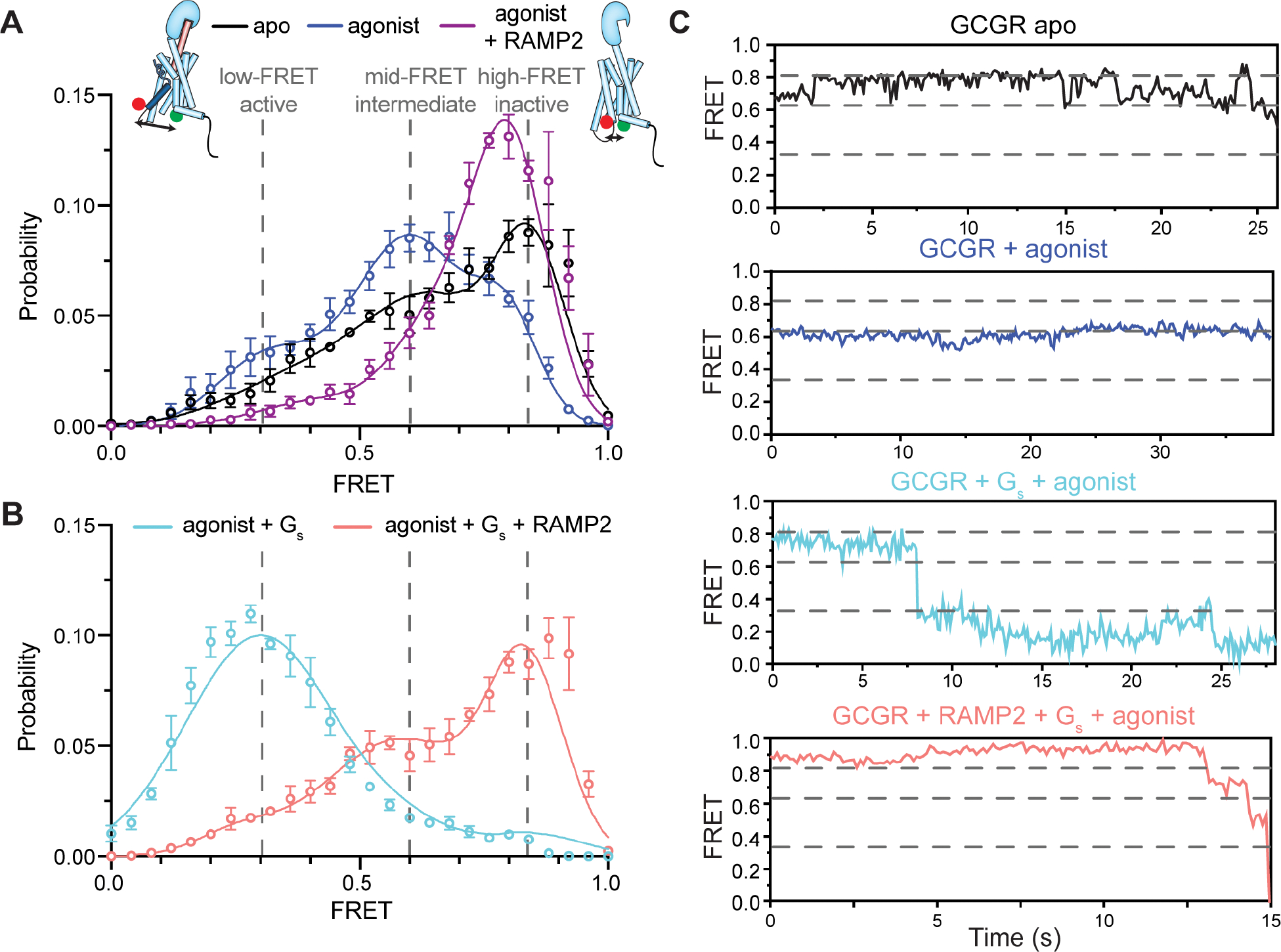
(A) smFRET experiments show that a dominant high-FRET state (~0.83) of GCGR labeled with donor and acceptor fluorophores at the intracellular ends of TM4 and TM6 is present in the absence of orthosteric agonist (A, black, N=162) with brief excursions to mid- (~0.63) FRET (~0.32) states (C, black), and the binding of agonist peptide results in a dominant mid-FRET state at the expense of the high-FRET state (A, blue, N=150; C). The mid-FRET intermediate and low-FRET active conformations of TM6 induced by agonist peptide are largely abrogated by the presence of RAMP2 (A, purple, N=117) as the distribution shifts towards and inactive-like conformation. Gs coupling to agonist-bound GCGR increases the population of the low-FRET, suggesting a full outward movement of TM6 at the expense of the mid-FRET agonist-specific intermediate state and high-FRET inactive state (B, cyan, N=158; C). However, pre-coupling of agonist-bound GCGR with RAMP2 potently inhibits a Gs-induced increase in the population of the fully outward TM6 conformation as well as the agonist-associated intermediate state (B, salmon, N=179; C). Histograms are shown with a 3-Gaussian fit to the data.
