Abstract
Urinary tract infections (UTIs) are among the most common infections and are associated with an increased rate of antimicrobial resistance in Saudi Arabia. Better knowledge of the most common pathogens and their antimicrobial resistance patterns will be useful for creating new treatment guidelines. PubMed, Web of Science, Scopus, and Google Scholar were searched using suitable keywords to identify UTI publications until November 2022. Eligible studies were selected and analyzed. A total of 110 records were found, but only 58 articles were analyzed. Most studies were retrospective, and just a few were cross-sectional or prospective. The majority of the studies were conducted in the central region followed by the Eastern region. Escherichia coli and Klebsiella spp. were the most common pathogens. There was a significant resistance rate against co-trimoxazole and ciprofloxacin. On the other hand, amikacin was one of the most effective antibiotics. Overall, only a few studies have been published on UTIs in Saudi Arabia. Moreover, not all regions have been represented, so the full scope of the issue is unknown. UTIs are still a major problem, and resistance has developed against commonly used antibiotics. Thus, large epidemiological studies are needed to battle the rapid emergence of antimicrobial resistance.
Keywords: UTI, urinary tract infections, uropathogens, Saudi Arabia, KSA
1. Introduction
Urinary tract infections (UTIs) are one of the most common types of infections [1]. They tend to be much more common in women; around 60% of women can expect to experience at least one UTI in their lifetime [2,3]. UTIs remain a burden for the healthcare system in the Kingdom of Saudi Arabia (KSA); they account for 10% of all infections in the country, and they stand as the second most common reason for emergency department admissions [2,3]. Around 4% of UTI patients are admitted to the hospital for further treatment. Another issue is readmission; around 10% are readmitted within one week of their discharge, and one of the main reasons is ineffective treatment [3]. Similar problems are faced around the world, as has been reported by several studies [4,5].
UTIs have usually been treated with broad-spectrum antibiotics. However, the treatment is often started without taking into consideration the bacterial culture or antimicrobial sensitivity patterns, which has led to the development of antimicrobial resistance worldwide. Nowadays, there is an alarming rate of antimicrobial resistance, leading to multidrug-resistant (MDR) bacteria [6]. Antimicrobial susceptibility patterns of the same bacteria can vary according to geographic location [5]. The Infectious Diseases Society of America recommends that regional surveillance should be conducted to monitor changes in the susceptibility of uropathogens in specific regions [7].
According to the European Union, the rate of human deaths related to antibiotic-resistant bacterial infections is approximately 25,000 per year, and two-thirds of these infections are due to Gram-negative bacteria [6,8]. One of the key factors contributing to the increased rate of antimicrobial resistance is overdiagnosis, which results in the overuse of antibiotics that might be unnecessary [9,10]. Hence, the diagnosis by urine culture is a necessity, especially for complicated UTIs, as it will confirm the infection and provide the physician with an antimicrobial pattern for that particular pathogen [4].
The main challenge in prescribing the treatment after confirming an infection by culture is time. According to the Saudi National Antimicrobial Therapy Guidelines, asymptomatic bacteriuria can be treated with empirical therapy without confirmation from a positive culture. Antimicrobials suich as nitrofurantoin, co-trimoxazole, ertapenem, and imipenem are among the first line of defense [10,11]. However, symptomatic bacteriuria should be treated after a confirmed microbiological culture, and it takes 2 to 3 days to receive a culture report in the microbiology lab [9,10]. International guidelines from the World Health Organization (WHO) suggest using nitrofurantoin and co-trimoxazole as the first line of defense. These treatments are also highly emphasized in the local guidelines [2,3]. Estimating the prevalence of the most commonly isolated pathogens and their antimicrobial resistance patterns is crucial for every hospital to avoid the emergence of new antibiotic resistance. Knowledge of the local antimicrobial sensitivity pattern is periodically required to plan an updated treatment regimen [12].
UTIs are very common, and bacterial antimicrobial resistance is emerging around the world, particularly in KSA. Most studies have been retrospective and descriptive in nature [13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41]. Most studies have tried to raise awareness of the emergence of antimicrobial resistance, but many of the data available are outdated [16,22,23,25,26,31,39,40,41,42,43,44]. Hence, periodic updates are needed to track this emergence. Moreover, no review papers have been published on this matter in KSA. This review is important as it will increase awareness of UTIs and antimicrobial resistance patterns in KSA [40,42,43,44]
As antimicrobial susceptibility patterns change rapidly, new resistant strains are emerging. Our review focused on the most common pathogens and their antibiotic susceptibility patterns. We hope this review can raise awareness among local healthcare professionals about emerging resistant strains, help them in their decisions to prescribe antibiotics, and be a tool to prevent and control the spread of resistant strains in the future.
2. Materials and Methods
2.1. Literature Review and Data Sources
The articles were found by searching PubMed, Web of Science, Scopus, and Google Scholar. All the studies published through November 2022 were included. Search keywords included urinary tract infection, uropathogen, UTI, Gram-negative bacteria, Gram-positive bacteria, antibiotics, antibiotic resistance, KSA, and Saudi Arabia. Additionally, national journals (Saudi Medical Journal and Annals of Saudi Medicine) and the reference lists of eligible articles were searched to identify additional published studies.
2.2. Eligibility Criteria
All published studies that (1) examined urinary tract infections and (2) were conducted in the Kingdom of Saudi Arabia were eligible for review (i.e., inclusion criteria). In addition, eligible studies had to include isolation and identification of UTI-causing uropathogens based on standard bacteriological methods following the Clinical Standards Laboratory Institute (CSLI) guidelines and using the approved automated systems or manual methods. Publications about UTIs that did not stem from primary research (e.g., opinion and letters to the editor), or were from conference proceedings or abstracts, were not included (i.e., exclusion criteria). All the studies were full-text and published in English.
2.3. Search Outcomes
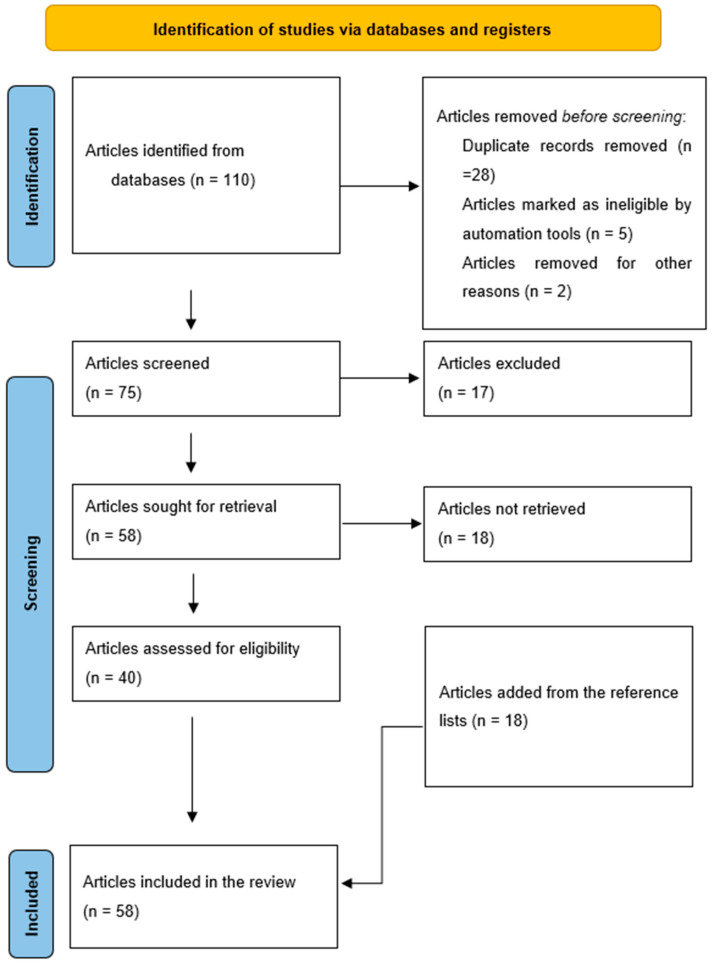
A total of 110 records were found. These produced 95 unique records after 28 duplicate articles were removed through a careful read of the study titles. Manuscripts were screened carefully, and only 40 met the inclusion criteria. An additional 18 articles were found while scanning eligible articles and their references, bringing the total to 58 articles for analysis (Figure 1).
Figure 1.
Flowchart of eligible articles.
2.4. Data Extraction Process and Analysis
From each included study, the following data were abstracted: (1) authors’ names, (2) publication year, (3) study design, (4) sample age and size, (5) study population, (6) location, and (7) main findings. Initially, the data of the included studies were charted by one co-author and then reviewed independently by the lead and senior authors. The main findings of the eligible articles were categorized under broad themes.
3. Results
3.1. General Description of the Studies
The majority of the studies were retrospective studies, and just a few were cross-sectional or prospective. The first study was published in 1988, and the following decades (i.e., 1991–2000 and 2001–2010) saw an approximately equal proportion of publications, whereas the last decade (i.e., 2011–2021) saw the most publications. Most studies were conducted in the central region, which includes Riyadh and other nearby cities, followed by the eastern region, Jeddah, and the Al Qassim region. Other regions had one or two publications per region. All age groups were included, from neonates to older adults (aged from 0 to +90 years). The sample sizes ranged from 82 [22] to 49,779 [27] participants (Table 1).
Table 1.
General description of included urinary tract infection (UTI) studies (n = 58).
| # | First Author, Year |
Design | Location | Sample Size | Patient Type | Age Range (Years)/Sex (M/F) | UTI Prevalence |
Most Common Pathogens |
Antibiotic Resistance |
Significant Correlates of UTI | Additional Comments |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Ahmed T. Eltahawy, 1988 [43] | Prospective | Jeddah | 575 | Patients presenting with urinary tract infection (UTI) | All ages (M/F) | 100% | E. coli, Klebsiella spp., Enterobacter spp., and Pseudomonas spp. | Sulfamethoxazole (78%), ampicillin (64%), tetracycline (62%) and carbenicillin (64%). | Not mentioned | Not mentioned |
| 2 | Ali Magzoub El-Bashier, 1991 [40] | Retrospective | Qatif | 13,193 | Patients with suspected UTI | All ages (M/F) | 7.6% | E. coli and Klebsiella spp., Enterobacter spp. | Ampicillin (70%), piperacillin (58%) | Not mentioned | Norfloxacin be considered an empirical therapy. |
| 3 | Hassan Abduljabbar, 1991 [44] | Prospective | Jeddah | 2642 | Pregnant patients presenting with suspected UTI | All ages (F) | 15.8% | E. coli and Klebsiella spp. | Not mentioned | Symptomatic group had a higher risk for premature birth (p < 0.05). | Hypertension and anemia in pregnancy were more common in women with bacteriuria. |
| 4 | D H Akbar, 2001 [25] | Retrospective | Jeddah | 182 | Patients presenting with UTI | All ages (M/F) | 100% | E. coli and Pseudomonas spp. | Not mentioned | Diabetes | Aminoglycoside and ciprofloxacin can be used empirically. |
| 5 | Abdulrahman A. Kader, 2001 [26] | Retrospective | Al-Khobar | 2394 | Patients presenting with UTI | All ages (M/F) | 100% | E. coli, Klebsiella spp. and Pseudomonas spp. | Trimethoprim (47%) and amoxycillin (62%). | Not mentioned | Not mentioned |
| 6 | Alia Abdulrahim Al-Ibrahim, 2002 [22] | Retrospective | Riyadh | 82 | Patients presenting with UTI | 0–5 (M/F) | 100% | E. coli and Klebsiella spp. | Not mentioned | Half of the patients in the study had bilateral reflux. | Hypertension scar formation and renal impairment were not detected. |
| 7 | Saeed M. Al-Asmar, 2004 [31] | Case-control | Riyadh | 824 | Inpatients | All ages (M/F) | 25% | Not mentioned | Not mentioned | UTIs account for one-third of nosocomial infections. | Not mentioned |
| 8 | Abdulrahman Abdulla Kader, 2004 [39] | Not mentioned | AL Khobar | 11,659 | Patients with suspected UTI | All ages (M/F) | 17.5% | E. coli, Klebsiella spp., Pseudomonas spp., Proteus spp. | Amoxicillin (61%) and trimethoprim (47%) | Not mentioned | There was noticeable antibiotic resistance. |
| 9 | Abdulrahman Abdulla Kader, 2005 [16] | Retrospective | Dammam | 2302 | Patients admitted with UTI | All ages (M/F) | 100% | E. coli, Klebsiella spp., Enterobacter spp. | Cefepime (88.5%), ciprofloxacin (86%) and gentamicin (77.5%) | Not mentioned | There is a high presence of ESBL producers in uropathogens among inpatients and outpatients. |
| 10 | Abdulrahman Abdulla Kader, 2005 [41] | Retrospective | Alkhobar and Dammam | 2455 | Patients presenting with UTI | All ages (M/F) | 100% | E. coli and Klebsiella spp. | ESBL-production (11%), cefepime (78%), ciprofloxacin (45%) | Not mentioned | It is important to apply measures to restrict the spread of ESBL infections. |
| 11 | Hanan H. Balkhy, 2006 [45] | Cross-sectional | Riyadh | 562 | Inpatients | All ages (M/F) | 38 (8%) | Not mentioned | Not mentioned | Hospital stay exceeding 8 days | Urinary catheters were reported as an important source of infection. |
| 12 | Abdulla A Al-Harthi, 2008 [23] | Retrospective | Aseer | 464 | Patients presenting with UTI | 0–12 (M/F) | 100% | E. coli, Klebsiella spp. and Pseudomonas spp. | Not mentioned | Not mentioned | Ceftriaxone, imipenem, and azactam are appropriate for initial empirical therapy. |
| 13 | Layla Alshamsan, 2009 [21] | Retrospective | Riyadh | 130 | Patients presenting with UTI | 0–12 (M/F) | 100% | E. coli, and Klebsiella spp. | Not mentioned | Not mentioned | Renal ultrasound has little value in the management of children with UTI. |
| 14 | Sameera M. Al Johani, 2010 [24] | Retrospective | Riyadh | 2792 | Patients presenting with UTI | All ages (M/F) | 100% | Acinetobacter baumannii, Pseudomonas spp., E. coli, Klebsiella spp. | Amikacin (94%), imipenem (90%), meropenem (90%) and ciprofloxacin (90%). | Not mentioned | Antimicrobial resistance is an emerging problem in the intensive care unit (ICU). |
| 15 | Khalid A Al-Rubeaan, 2013 [46] | Cross-sectional | Riyadh | 1000 | Diabetic patients | All ages (M/F) | 25.3% | Not mentioned | Not mentioned | The incidence of UTI in both type 1 and 2 diabetics was similar. | The body mass index, hypertension, microalbuminuria and insulin therapy were significantly higher in patients with UTI. |
| 16 | DA Abdulmutalib, 2013 [47] | Prospective | Taif | Not clearly specified | Inpatients | All ages (M/F) | Not clearly specified | E. coli and Pseudomonas spp. | Not mentioned | Not mentioned | Catheter associated urinary tract infections (CAUTIs) declined from 3.5 to 2.2 per 1000 catheter-days in 2013. |
| 17 | Jaffar A. Al Tawfiq, 2013 [48] | Prospective | Dhahran | Not clearly specified | Inpatients | All ages (M/F) | Not clearly specified | Not mentioned | Not mentioned | CAUTI was the most common nosocomial infection. | The use of preventive bundles was effective in decreasing the infection cases. |
| 18 | Md. Afzal Hossain, 2013 [49] | Cross-sectional | Riyadh | 510 | Patients presenting with UTI | All ages (M/F) | 100% | E. coli, Klebsiella spp. and Pseudomonas spp. | Ampicillin (84%), cephalothin (75%), and co-trimoxazole (62%). | Not mentioned | Ciprofloxacin resistance was also closely associated with multidrug resistance. |
| 19 | Abdulla A. Alharthi, 2014 [50] | Cross-sectional | Taif | 1000 | Outpatients | 3–6 (M/F) | 5% | E. coli and Enterococci | Not mentioned | 25% of the screened children had urinary abnormalities. | Pyuria were evident in 5% of cases and hematuria in 2.5%. |
| 20 | Mansoor Sirkhazi, 2014 [34] | Retrospective | Dammam | 106 | Neutropenic cancer patients | All ages (M/F) | 29.71% | E. coli, Klebsiella spp., Staphylococcus aureus | Not mentioned | Cancer patients with febrile neutropenia | The use of initial antibiotic therapy in febrile neutropenic episodes should be based on local bacterial spectrum. |
| 21 | Mohamed H Al-Agamy, 2014 [38] | Retrospective | Riyadh | 152 | Patients presenting with UTI | All ages (M/F) | 100% | E. coli | ESBL-production 20% | Not mentioned | Not mentioned |
| 22 | Menyfah Alanazi, 2015 [2] | Cross-sectional | Riyadh | 5752 | Patients presenting in emergency department | All ages and (M/F) | 24.9% | E. coli and Klebsiella spp. | Penicillin and cephalosporin showed the highest resistance rates, but data was not provided. | Not mentioned | Penicillin and cephalosporin were the most common wrongly prescribed antibiotics. |
| 23 | TA El-Kersh, 2015 [51] | Not mentioned | Khamis Mushayt | 269 | Patients presenting with UTI | All ages (M/F) | 100% | E. coli and Klebsiella spp. | Co-trimoxazole (53%) and nitrofurantoin (25%) | Not mentioned | Not mentioned |
| 24 | Wallaa A. Garout, 2015 [20] | Retrospective | Riyadh | 153 | Patients presenting with UTI | 0–5 (M/F) | 100% | E. coli, followed by Klebsiella spp. | Not mentioned | A single episode of UTI signified normal urological anatomy. | Urological anomalies were found in 28.1% of the overall study population. |
| 25 | Hani S. Faidah, 2015 [52] | Prospective | Mecca | 200 | Pregnant patients presenting with UTI | 18 to 45 years (F) | 100% | E. coli and Klebsiella spp. | Ampicillin (55%) and tetracycline (34%) | UTI is very common among pregnant women in Mecca. | Not mentioned |
| 26 | Sulaiman Ali Al Yousef, 2016 [53] | Cross-sectional | Hafr Al Batin | 908 | Patients with suspected UTI | All ages (M/F) | 75% | E. coli and Klebsiella spp. | Ampicillin (90%), mezlocillin (88%) and co-trimoxazole (66%) | Not mentioned | High resistance to commonly prescribed empirical therapy was observed. |
| 27 | Mohamed S. Kabbani, 2016 [54] | Retrospective | Riyadh | 413 | Inpatients | Children who underwent cardiac surgery (M/F) | 7% | Klebsiella spp. and E. coli | 33% of the pathogens were multidrug resistant. | Long duration of catheters | Resistant Gram-negative bacteria are an emerging concern in ICUs. |
| 28 | Samiah HS Al-Mijalli, 2017 [55] | Prospective | Riyadh | 116 | Patients presenting with UTI | All ages (M/F) | 100% | E. coli, Klebsiella spp. and Pseudomonas spp. | Imipenem (98%), meropenem (98%) and ampicillin (95%) | Not mentioned | Pathogens were susceptible to meropenem, imipenem, colistin, and ertapenem. |
| 29 | Fahad M. Al-Hameed, 2018 [56] | Prospective | Jeddah | Not clearly specified | Inpatients | All ages (M/F) | Not clearly specified | Not mentioned | Not mentioned | Not mentioned | The monthly rates of CAUTI significantly declined after the enforcement of preventive strategies. |
| 30 | Eiman Gaid, 2018 [57] | Prospective | Different hospitals from different regions | 6178 | Inpatients | All ages (M/F) | 28.4% | Not mentioned | Not mentioned | Not mentioned | CAUTI occurred from 2.3 to 4.4 per 1000 device-days. |
| 31 | Osama Al Wutayd, 2018 [58] | Cross-sectional | Buraidah | 418 | Patients presenting with UTI | All ages (M/F) | 100% | E. coli, Klebsiella spp., Proteus mirabilis and Pseudomonas spp. | Ampicillin (89%), oxacillin (75%), and piperacillin (85%). | Not mentioned | There is a high multidrug resistance rate. |
| 32 | Bander Balkhi, 2018 [13] | Retrospective | Riyadh | 1918 | Patients presenting with UTI | All ages and (M/F) | 100% | E. coli, Klebsiella spp. and Pseudomonas spp. | Co-trimoxazole (47%) followed by ciprofloxacin (34%) | Diabetes and pregnancy | The development of regional and national UTI guidelines is recommended. |
| 33 | Menyfah Q Alanazi, 2018 [32] | Retrospective | Riyadh | 1449 | Patients visiting emergency department | All ages (M/F) | 9.9% | E. coli | Not mentioned | Not mentioned | There is a significant level of inappropriate use of antibiotics in the treatment of UTIs in the emergency department. |
| 34 | Abdulaziz Alqasim, 2018 [37] | Retrospective | Riyadh | 100 | Patients presenting with UTI | All ages (M/F) | 100% | E. coli | ESBL production (67%) ampicillin (92%) and to amoxicillin–clavulanic acid (55%). | Not mentioned | 67% of ESBL were multidrug resistant (MDR). |
| 35 | Menyfah Q. Alanazi, 2018 [33] | Retrospective | Riyadh | 565 | Patients admitted with UTI in emergency department | All ages (M/F) | 100% | E. coli | Ampicillin (35%) and co-trimoxazole (43%) | Not mentioned | Higher resistance rate was noticed in young patients <12 years. |
| 36 | Salem K. Albalawi, 2018 [59] | Not mentioned | Tabuk | 210 | Patients presenting with UTI | 0–12 (M/F) | 100% | E. coli and Klebsiella spp. | Ampicillin (87%) and cotrimoxazole (81%) was observed. | Not mentioned | For E. coli the lowest resistance rate was for nitrofurantoin. |
| 37 | Abdulaziz Alamri, 2018 [27] | Retrospective | Aseer | 49,779 | Patients presenting with UTI | All ages (M/F) | 100% | E. coli and Klebsiella spp. | Cephalothin (90%), nalidixic acid (87 %), and ampicillin (82%). | Not mentioned | Not mentioned |
| 38 | Ibrahim Taher, 2019 [60] | Retrospective | Aljouf | 415 | Patients presenting with UTI | All ages (M/F) | 100% | E. coli, Klebsiella spp., and Pseudomonas spp. | Ampicillin (84%) and co-trimoxazole (53%). | Not mentioned | There is a high incidence of MDR strains. |
| 39 | Sulaiman I. A. Alsohaim, 2019 [29] | Retrospective | Buraidah | 379 | Patients presenting with UTI | All ages (M/F) | 100% | E. coli, Klebsiella spp., and Pseudomonas spp. | Cefoxitin (71%) and gentamicin (48%). | Not mentioned | There was significant negative relationship between antimicrobial prescribing and resistance. |
| 40 | Syed Suhail Ahmed, 2019 [30] | Retrospective | Buraidah, Qassim | 273 | Patients presenting with UTI | All ages (M/F) | 100% | E. coli, Klebsiella spp., and Proteus spp. | Ampicillin (88%), piperacillin (72%), clindamycin (66%) and amoxicillin/clavulanic acid (66%). | Not mentioned | There is high incidence of multidrug-resistant strains. |
| 41 | Hameed T, 2019 [15] | Retrospective | Riyadh | 202 | Patients admitted with UTI | Pediatric patients 0–14 (M/F) | 100% | E. coli followed by Klebsiella spp., Pseudomonas spp. and Enterococcus | Ampicillin (68%) and co-trimoxazole (54%). | Not mentioned | For children with a community-acquired UTI, a third-generation cephalosporin is a safe choice. |
| 42 | Majid M. Alshamrani, 2019 [61] | Not mentioned | Different hospitals from different regions | 1666 | Inpatients | All ages (M/F) | 6.8% | Not mentioned | Not mentioned | Not mentioned | Hospital-acquired UTIs accounted for 20% of all nosocomial infections. |
| 43 | Nehad J. Ahmed, 2021 [62] | Retrospective | Alkharj | 7703 | Inpatients | All ages (M/F) | 0.5% | Not mentioned | Not mentioned | The rate of overall healthcare-associated infections was low. | The compliance rate to preventive measures was high. |
| 44 | Abdulrahman S Bazaid, 2021 [36] | Retrospective | Ha’il | 428 | Patients presenting with UTI | All ages (M/F) | 100% | E. coli, Klebsiella spp., and Staphylococcus aureus | Piperacillin (45%) and co-trimoxazole (40%). | Not mentioned | Carbapenem and linezolid can be considered first therapeutic choices. |
| 45 | Yaser Saleh Bamshmous, 2021 [19] | Retrospective | Jeddah | 278 | Patients presenting with UTI | 0–16 (M/F) | 100% | Staphylococcus, followed by Klebsiella spp. | Data was not provided | Not mentioned | Not mentioned |
| 46 | Mohammed Abdullah Alzahrani, 2021 [17] | Retrospective | Al-Baha | 118 | Patients admitted with UTI | Pediatric patients 0–14 (M/F) | 100% | E. coli, ESBL E. coli, Klebsiella spp., Enterococcus faecalis | Ampicillin (94%), cephalothin (92%), and cefoxitin (76%). | Not mentioned | Antibiotic resistance can be reduced compliance to guidelines. |
| 47 | Mohammed Yahia Alasmary, 2021 [18] | Retrospective | Najran | 136 | Patients presenting with UTI | All ages (M/F) | 100% | E. coli, and Klebsiella spp. | Ampicillin (63%) and cephazolin (60%). | Not mentioned | The patients with UTIs in the Najran region of KSA are at a high risk of antibiotic resistance. |
| 48 | Mariam Alrasheedy, 2021 [63] | Cross-sectional | Ministry of Health hospitals in KSA | 1083 | Patients with suspected UTI | 0–10 year sold (M/F) | 25.8% | E. coli, Proteus, Klebsiella spp., Enterococcus, Citrobacter | Not mentioned | Not mentioned | Nearly a sixth of children could develop severe/complicated UTI. |
| 49 | Abdulaziz Alamri, 2021 [12] | Retrospective | Abha | 1506 | Patients presenting with UTI | All ages and (M/F) | 100% | E. coli and Klebsiella spp. | Ampicillin (91%) and cephalothin (93%). | Not mentioned | Fosfomycin, cefoxitin, nitrofurantoin, are recommended as first-line treatment. |
| 50 | Lina Almaiman, 2021 [14] | Retrospective | Buraidah | 754 | Outpatients of nephrology clinic | 14–95 (M/F) | 21.8% | E. coli and Klebsiella spp. | Not mentioned | Chronic kidney disease (CKD). | The management of comorbidities could help to control the progression of CKD to the late stages. |
| 51 | Saad Alghamdi, 2021 [35] | Retrospective | Mecca | 678 | Cancer patients | All ages (M/F) | 44% | Klebsiella spp., E. coli and Pseudomonas spp. | Fluoroquinolones (50%), cephalosporin (50%) and carbapenems (50%). | Not mentioned. | Enhanced antibiotic resistance was found by Gram-negative bacilli. |
| 52 | Kawther Aabed, 2021 [64] | Prospective | Riyadh | 113 | Patients presenting with UTI | All ages (M/F) | 17.5% | E. coli | Norfloxacin (80%), amoxicillin (70%) ampicillin (70%) and co-trimoxazole (55%). | Not mentioned | Not mentioned |
| 53 | Samiah Hamad S Al-Mijalli, 2022 [65] | Cross-sectional | Riyadh | 100 | Type 2 diabetes patients | All ages (M/F) | 22% | Streptococcus, and Pseudomonas spp. | Tigecycline (88%), gentamycin (84%) and nitrofurantoin (78%). | Diabetic patients are prone to a wide range of pathogens. | Tigecycline can be used as empirical treatment. |
| 54 | Bader S Alotaibi, 2022 [28] | Retrospective | Aljouf | 1334 | Patients presenting with UTI | All ages (M/F) | 100% | E. coli, Klebsiella spp., and E. faecalis | Carbapenems (37%) and) to meropenem (34%). | Not mentioned | MDR gram-negative bacteria dominate the pathogenic spectrum of UTI. |
| 55 | Mohd Saleem, 2022 [66] | Cross-sectional | Ha’il | 1078 | Inpatients | Older than 18 years (M/F) | 6.5% | Klebsiella spp. | Mupirocin (80%), tigecycline (80%) and ceftriaxone (47%). | Not mentioned | It is recommended that a smaller-size catheter be used to provide better drainage. |
| 56 | Yvonne S. Aldecoa, 2022 [67] | Prospective | Different hospitals | 919,615 patient-days | Patients with a urinary catheter | All ages (M/F) | 965 cases | Not mentioned | Not mentioned | Not mentioned | CAUTI rate was 1.68 per 1000 urinary-catheter days. |
| 57 | Sarah Alrashid, 2022 [3] | Retrospective | Riyadh | 315 | Patients presenting with UTI | All ages (M/F) | 100% | E. coli, Klebsiella spp., and Pseudomonas spp. | Ampicillin (58%) and co-trimoxazole (42%). | Not mentioned | High resistance was found against antibiotics used as empirical therapy. |
| 58 | Adil Abalkhail, 2022 [68] | Randomized experimental study | Riyadh | 2250 | Patients presenting with UTI | All ages (M/F) | 100% | E. coli | Ampicillin (100%) cephalosporins (90%) ESBL-production (33%). | Not mentioned | High resistance was found against antibiotics used as empirical therapy against ESBL. |
CAUTI: catheter-associated urinary tract infection; CKD: chronic kidney disease; ESBL: extended-spectrum β-lactamases; E. coli: Escherichia coli; ICU: intensive care unit; UTI: urinary tract infection.
3.2. UTI in Diabetic Patients
Different comorbidities such as diabetes and pregnancy are associated with complications such as urinary tract infections [69]. Five studies specifically mentioned the UTI prevalence in diabetic patients [13,14,25,46,65]. A study conducted in Riyadh found that diabetes was one of the main causes of complicated UTIs, which extends beyond the bladder to the upper urinary system [13]. In another study, it was reported that 25.3% of the general diabetic population experienced UTIs [13]. Female patients were at much higher risk compared to men, even among patients who did not have diabetes or other chronic conditions [13,25]. There were other risk factors that were associated with an increased risk for developing UTI among diabetic patients, such as hypertension (p = 0.006), microalbuminuria (p = 0.031), insulin therapy (p < 0.001), and a body mass index (BMI) greater than 30 kg/m2 (p < 0.001), all of which are complications of diabetes [14,46]. Other factors such as age, diabetes type, duration of diabetes, and HbA1C levels did not significantly increase the risk of developing a UTI [46]. Diabetic patients are at high risk of asymptomatic pyuria, bacteriuria, and upper urinary tract infections. One of the main factors is glucosuria, as glucose is used as a source of energy for bacteria, thus creating a suitable environment for bacteria to replicate at a high rate; it is also related to neutrophil dysfunction [13,25].
The most common isolated pathogens were Escherichia coli, Pseudomonas, and Staphylococcus hominis. Only one study reported Staphylococcus hominis as the most common pathogen [64]. All groups showed a high resistance rate to ampicillin and clindamycin. A lower resistance was reported to imipenem, meropenem, and amikacin. Imipenem is one of the alternative drugs advised by the Saudi National Antimicrobial Therapy Guidelines [11]. There were high rates of sensitivity to aminoglycoside, tigecycline, gentamycin, and ciprofloxacin. Most of the isolated pathogens were reported to have been multidrug resistant [13,25,64]. Ciprofloxacin was suggested as the drug of choice for empirical therapy in 2001, and, still in 2022, another study reported high bacterial sensitivity to it, indicating that it is a safe choice for many clinicians. The rate of resistance to ciprofloxacin was reported as high as 34% [13,25].
3.3. UTI in Older Adults
UTIs associated with asymptomatic bacteriuria are a common challenge in older patients. This is due to the lack of localized genitourinary symptoms [70]. There were three papers in which older adults were specifically studied [32,33,65]. One of the studies reported that older patients were more prone to UTIs; 37.5% of urine cultures from older patients were positive for growth as compared to 24.34% and 32.29% in adults and children, respectively [33]. UTIs accounted for 14.6% of emergency department visits by older patients in 2018 and 11% in 2001 [32,65]. The prevalence of UTI was reported to be more common among female patients, but it tended to decrease with age. On the other hand, the prevalence among males tended to increase with age, especially among older men [33].
E. coli, Klebsiella species, and Enterobacter species accounted for most infections. There was a high resistance rate to ampicillin and trimoxazole commonly found among isolates [33,65]. UTI pathogens showed a higher resistance rate to antibiotics such as amoxicillin–clavulanic acid, ciprofloxacin, and nitrofurantoin among patients older than 65 years of age. Moreover, 47.2% of older patients experienced inappropriate antibiotic prescriptions, with inappropriate duration of treatment being the most common error (p < 0.05) [33]. Multidrug resistance was much more common among older people (50%) compared to other age groups (<20%), as was extended-spectrum β-lactamases (ESBL)-producing E. coli (8% in older people vs. 5% in other age groups) [32]. This high rate of resistance suggests the need to reevaluate and have an updated empiric therapy to combat the emergence of new resistant strains [32,33].
3.4. UTI in Cancer Patients
Two papers studied UTI prevalence in cancer patients. UTIs were the second most common infection after bloodstream infections [34,35]. The most common malignancies among the study groups were non-Hodgkin’s lymphoma and AML (acute myeloid leukemia), followed by colorectal cancer and other types of malignancies. Patients who undergo chemotherapy have a high risk of developing febrile neutropenia, characterized by a fever of more than 38 °C and a decreased neutrophil count (less than 500 cells/mm3), which is also associated with an increased risk for infection and mortality. Bacterial infections are among the major causes of mortality in neutropenic cancer patients [34,35].
In the past decades, there has been a shift in the prevalence of bacterial infections, from Gram-positive to Gram-negative being the most common. Studies conducted in the last decade reported E. coli and Klebsiella spp. as the most common Gram-negative bacteria pathogens [34,35]. Both pathogens were susceptible to imipenem-cilastatin, amikacin, piperacillin-tazobactam, and ceftriaxone. The ESBL production rate for E. coli and Klebsiella spp. were 38% and 22%, respectively [34,35].
3.5. UTI in Emergency Department Patients
UTIs are among the most common causes of emergency department (ED) visits, and the most common medical condition for which antibiotics are prescribed in many places around the world [71]. Four papers were found that included cases with UTI from emergency department [2,3,32,33]. UTI cases accounted for around 10% of total visits to the ED, with a higher prevalence of 13.3% in the month of January. Regarding age groups, 14.6% of older adults visited EDs due to UTI symptoms [3,32].
The male-to-female ratio was reported as 1:2 [3,32]. Women were most commonly affected by UTIs, except among older adults, where female patients were less than 50% of total older patients presenting with a UTI [3,33]. The average hospital stay of a patient with a UTI was around 3.5 days, and the longest stay was 23 days. Around 24% of patients required isolation, and 3% required admission to the ICU (intensive care unit).
E. coli was the most frequently isolated pathogen, followed by Enterobacter spp., Klebsiella spp., and Acinetobacter spp. E. coli showed resistance to ampicillin and co-trimoxazole and showed sensitivity to nitrofurantoin, followed by ciprofloxacin, amoxicillin–clavulanic acid, and cefazolin. ESBL-producing E. coli was detected in approximately 7% of the isolates [2,32,33].
Most of the patients (around 80%) were initially treated with broad-spectrum antibiotics. Cephalosporin and penicillin were the most common classes. Cefuroxime and norfloxacin were the most commonly prescribed antibiotics among adult and older adult patients, whereas in pediatric patients, the most common choice was amoxicillin–clavulanic acid followed by cefprozil [2,32,33].
UTIs account for a high economic cost for emergency departments. Only one study reported the economic impact of UTIs on EDs [32]. The cost of hospital treatment for a UTI in the ED of King Abdulaziz Medical City in 2018 ranged from USD 90.19 to USD 328.65 per patient, and an estimated yearly cost of USD 838,375. Another factor that contributes to the high cost is inappropriate prescription of antibiotics, which accounted for 47% of the overall cost (p < 0.05).
To some extent, we can say that there was an overestimation of UTIs among patients who visited EDs with UTI-like symptoms. This was confirmed with a 60% prevalence of positive cultures [3], and in some other cases only 30% of total ordered urine cultures from the ED were positive [32,33].
Another problem that was noted was the burden of readmissions. Around 10% to 15% of patients were found to be readmitted to the ED within 30 days after discharge, and the median time for readmission was around 7 days. Patients who required isolation were more likely to get readmitted. Readmission was more common in patients having underlying diseases [3]. Unplanned readmission is a huge burden on the hospital facilities, especially for emergency departments, as it increases the cost and overloads the setting.
3.6. Inappropriate Antibiotic Prescriptions
The overuse and inappropriate use of antibiotics are two of the most important causes of antimicrobial resistance [72]. According to a report from the WHO, around 20% to 50% of antibiotics are inappropriately prescribed [2,6]. Only two papers identified this issue in KSA [2,32]. Inappropriate use of antibiotics includes dose errors, duration errors, frequency errors, and inappropriate selection of antibiotic class [2,32]. Around 47.3% of prescribed antibiotics in EDs were inappropriate. Pediatric patients were the most vulnerable group (p < 0.001); 57.8% were prescribed inappropriate antibiotics as compared to a rate of 37.8% among adult patients. Pediatric patients were more prone to errors such as dosage (p < 0.001) and duration errors (p < 0.001), while adult patients were more prone to errors such as inappropriate selection of antibiotic class (p < 0.001). However, neither study reported a significant association between age and frequency of selection error (p = 0.74) [2,32].
Inappropriate antimicrobial prescription for a UTI is more common in the ED as EDs often prescribe antibiotics. Using a lower concentration of antibiotic or for a shorter duration will cause antibacterial resistance, and overuse will result in toxicity, an increased antimicrobial resistance rate, or other side effects. Inappropriate prescription is also associated with several clinical manifestations such as allergies, gastrointestinal disturbances, renal/liver distress, etc. Assessing the weight of an infant before prescribing a dosage of any antibiotic is quite important. Studies show that even though the weight of the infant was measured, there were still errors in dosage calculation, with overdose being the most common calculation error [2,32].
Inappropriate selection of antibiotics is related to an increased antibiotic resistance rate, along with several complications and an increased chance for readmission. It will also affect the healthcare system as it will increase the cost of treatment and make the battle against antimicrobial resistance more difficult. There was an interesting correlation between diagnostic tests, prescription inappropriateness, and cost. Patients who had undergone diagnostic tests had a higher incidence of being prescribed expensive antibiotics. On the other hand, patients who did not undergo diagnostic tests initially had a lower cost, but they were inappropriately prescribed antibiotics, causing many later complications [2,32].
Cephalosporin was 3.31 times more likely to be inappropriately prescribed to adult patients, compared to penicillin prescriptions. On the other hand, penicillin was more likely to be inappropriately prescribed to pediatric patients (33.6%) compared to other antibiotics (p < 0.05). Cephalosporin was the most inappropriately prescribed antibiotic (more than 90%). Most of the patients who were administered or prescribed antibiotics were not screened for antibiotic allergy; only 7% of patients were screened for antibiotic allergy prior to its administration or prescription.
3.7. Prevalence of ESBL
Thirteen articles were found that mentioned and tested ESBL-producing uropathogens. They were detected in approximately 2% [39], 7% [32], 8% [26], 9% [16], 11% [41], 12% [17], 15% [13], 17% [64], 20% [38], 45% [37], 33% [68], 43.5% [23], and 44% [51] of urinary specimens. ESBL species have been a major concern for healthcare systems all around the world since 2000 when their outbreak started [16,68].
ESBL-producing E. coli are much more prevalent among women than men. ESBL-producing E. coli were resistant to gentamicin, ceftazidime, amoxicillin–clavulanic acid, cefotaxime, levofloxacin, and ciprofloxacin, and they showed sensitivity towards carbapenems, amikacin, and piperacillin/tazobactam [18,19,21,23,24,30]. ESBL-producing Klebsiella spp. showed resistance to piperacillin, cefotaxime, and ceftazidime and sensitivity towards piperacillin-tazobactam, amikacin, and carbapenems [14,18,41].
3.8. UTI in Children
Twelve papers reported UTIs in children (0–12 years old) [2,15,17,19,20,21,22,32,33,50,59,62]. UTIs account for 4% to 15% of total ED visits by pediatric patients [2,32]. UTIs and related complications account for around 5% of ED visits by children, compared to 15% of ED visits by other age groups. UTIs in children are common but difficult to diagnose due to their ambiguous nature. On the other hand, they have a very good prognosis [15,50]. Girls are at higher risk for infection. Up to the age of 6 years, the incidence rate is similar for both sexes, and this could be due to hygiene or other causes, but later the incidence among boys starts to decrease [15,21,50]. Circumcision is significantly associated with the development of UTIs among young boys. Uncircumcised boys are more prone to UTI than circumcised boys [20].
Some studies performed renal ultrasound on pediatric patients with a confirmed UTI. The most commonly reported abnormalities were hydronephrosis, uretric dilatation, dilatation of the renal calices and dilatation of the collecting system. Patients with abnormal ultrasound findings were associated with repeated episodes of UTI as compared to patients with normal findings who had only a single episode (p < 0.05). Moreover, abnormal ultrasound findings were significantly more common in non-E. coli UTIs [20,21,22].
As UTIs are common among children, they can be severe and have serious complications. A common complication is vesicoureteral reflux. Renal ultrasound is a good method to detect this complication [20,21,22]. Vesicoureteral reflux can be found in around 30% of children affected by UTI and bilateral reflux was very common as well. Patients who had vesicoureteral reflux had a high risk of developing pyelonephritis and scarring. In addition, renal parenchymal scarring was reported as a common complication of delayed treatment [20,21,22].
E. coli was reported as the most common isolated organism, followed by Klebsiella spp., E. faecalis, and methicillin-resistant Staphylococcus epidermidis (MRSE) [2,15,17,19,20,21,22,32,33,50,59,62]. Non-E. coli infections were found to be more common in young boys (p < 0.0001), but overall, young girls were much more prone to UTI [15].
E. coli showed resistance against ampicillin, cephalothin, co-trimoxazole, and cefoxitin and was sensitive to cefotaxime, ceftazidime, colistin, levofloxacin, and tigecycline [2,15,17,19,20,21,22,32,33,50,59,62].
Klebsiella spp. were highly resistant against ampicillin, cefoxitin, cefuroxime, cephalothin, and tobramycin. It showed less resistance to co-trimoxazole. Klebsiella spp. were highly sensitive to amikacin, aztreonam, cefepime, ciprofloxacin, gentamicin, imipenem, and levofloxacin, followed by meropenem, nitrofurantoin, norfloxacin, ceftriaxone, tazocin, and amoxicillin/clavulanic acid [17,18,23].
E. faecalis was not a commonly isolated pathogen. It was found to be highly sensitive to cephalothin, ciprofloxacin, linezolid, nitrofurantoin, teicoplanin, and vancomycin, but it was highly resistant to cefepime and tetracycline, followed by clindamycin and erythromycin, cefoxitin, gentamicin, and ampicillin [17].
Inappropriate antibiotic prescriptions were most common among pediatric patients, accounting for 51.3% of all prescriptions [16]. Some recent studies suggest the use of third-generation cephalosporin as a broad spectrum for community-acquired UTI because the majority of uropathogens were sensitive to it (96%) [17,19].
3.9. UTI in Adults
Urinary tract infections are among the most common infections worldwide, and they affect people from all age groups, especially women. The WHO has reported that an estimated 50% of women experience a UTI at some point in their lives [18]. We found 18 papers that studied UTIs in adults, especially in women, as they experienced more infections than men [2,13,18,24,26,27,28,29,30,32,33,36,40,43,49,55,58,60]. Adult patients were the most represented in all the studies. In KSA, UTIs were reported to be the second leading cause of infection (predominantly in women) at EDs. Adult patients account for the most visits to an ED presenting with symptoms of UTI [2]. The vast majority of healthy adults who have a UTI will present with mild symptoms and can recover from the infection with a simple medication plan. The problem is reoccurrence, and using ineffective antibiotics will lead to an increased rate of resistance and urinary tract-related complications. On a large scale, many of the studies reported female patients as more likely to present with recurring UTIs [2,36,55].
The most commonly isolated organisms were E. coli, followed by Klebsiella spp., P. aeruginosa S. agalactiae, E. faecalis, Streptococcus group B and D, methicillin-resistant Staphylococcus Aureus (MRSA), and vancomycin-resistant E. faecalis (VRE). Some studies even reported a significantly higher prevalence of UTI caused by E. coli among women (p < 0.05) [2,13,18,24,26,27,28,29,30,32,33,36,40,43,49,55,58,60].
E. coli isolates showed a high resistance rate to ampicillin, co-trimoxazole, mezlocillin, piperacillin, trimethoprim, ciprofloxacin, and to cefazolin. On the other hand, they showed a high sensitivity rate to amikacin, ertapenem, nitrofurantoin ciprofloxacin, levofloxacin, moxifloxacin imipenem, meropenem, norfloxacin, and piperacillin/tazobactam [2,13,18,24,26,27,28,29,30,32,33,36,40,43,49,55,58,60].
Klebsiella spp. was the second most common pathogen, and it showed the highest resistance rate to ampicillin co-trimoxazole, followed by cefuroxime, aztreonam, cefepime, ceftriaxone, cefuroxime, cephalothin, ceftazidime, and amoxicillin. Additionally, Klebsiella spp. showed a high susceptibility rate to meropenem, imipenem, colistin, ertapenem, amikacin, and levofloxacin. The antimicrobial resistance patterns of other less common pathogens were similar to E. coli and Klebsiella spp. Ertapenem and imipenem are the first line of defense against ESBL-producing Enterobacter according to the Saudi National Antimicrobial Therapy Guidelines [11].
3.10. UTI in Pregnant Women
UTIs are very often encountered among women, especially pregnant women [73]. Three papers reported on UTIs in pregnant women [13,44,52]. The first one was published in 1991 [44], a second in 2013 [52], and the most recent in 2018 [13].
In the 1991 study [44], the prevalence of UTI among pregnant women was 16%. However, in the study that was conducted in the last decade, the prevalence of UTI was as high as 20% [52]. In both studies, around half of the patients who tested positive for UTI were asymptomatic. Patients with symptomatic UTI were significantly more likely to have a premature birth as compared to the asymptomatic group [44]. Asymptomatic UTIs can spread to the upper renal system and cause pyelonephritis, which is a major cause of septic shock among pregnant women [44,52].
UTIs during pregnancy were reported to be a common problem and they were commonly associated with several disorders [44,52]. Pre-eclampsia (a high blood pressure disorder that can occur during pregnancy) tended to be more common among patients with UTI. There was also a significant relationship between the presence of bacteriuria and development of conditions such as anemia and hypertension [44]. The incidence rate was similar between symptomatic and asymptomatic women [44,52].
The most common causative agents of UTI among pregnant women were E. coli, Staphylococcus, and Candida species, which is an indication of fecal contamination and poor personal hygiene [44,52].
Amoxicillin, cefoxitin, ceftazidime, norfloxacin, penicillin, and fusidic acid were the most useful antibiotics for treating UTIs as they showed the least resistance frequency. On the other hand, ampicillin and tetracycline had the highest resistance [13,44,52].
3.11. Catheter-Associated UTIs
Catheter-associated urinary tract infections (CAUTIs) are among the most frequent hospital-acquired infections. There were around eleven publications on CAUTIs [45,47,48,54,56,57,61,62,66,67,74]. CAUTIs are the most common nosocomial infections in KSA, where they account for 7% [54], 20% [61] 22% [66], 24.4% [45], 28.5% [57], and 42% [48] of hospital-acquired infections. There are several risk factors for the occurrence of CAUTI among ICU patients, such as prolonged hospitalization, prolonged catheter usage, poor aseptic technique, inappropriate use of antibiotics, gender, extremes of age, immunosuppressive drugs, etc. Sixty to seventy percent of CAUTIs can be prevented by proper infection control and by implementing preventive bundles [67]. Another risk factor related not only to CAUTIs but also to nosocomial infections in general is that they are caused by pathogens with very strong resistance rates and are a huge challenge to treat, especially for immunocompromised patients [45,54,61].
CAUTIs were more common in older patients (60 to 80 years old) [66,74]. The main causative pathogens were Klebsiella spp., followed by E. coli and Proteus, with a high sensitivity to amikacin, gentamicin, and carbapenem [54,66]. However, most of the articles did not report the main pathogens or their antimicrobial resistance patterns.
Some studies reported on interventions over a long period of time and tried to reduce the rate of nosocomial infections. Before the intervention, the rate of CAUTIs among patients admitted to ICU was 2.3 per 1000 days. After initial screening of the catheter and patients who were catheterized in ICU and with proper documentation and discouragement of routine replacement, the infection rate dropped to 1.9 per 1000 days in a short period [45]. In another intervention that lasted 2 years, the CAUTI rate dropped from 3.5 per 1000 days before the intervention to 2.9 per 1000 days after 1 year, to 2.2 in the beginning of the second year, and to 1.7 at the end of the last year [56]. In a 7-year surveillance and intervention, the CAUTI rate dropped from 6.75 per 1000 days to 3.41 per 1000 days [48]. The CAUTI rate dropped significantly after the intervention, but there is another noticeable fact. The early 2000s study [48] reported an incidence rate of around 10 per 1000 days, and the most recent study reported an incidence rate as low as 0.3 per 1000 days [56]. This is an indication that the standard of healthcare facilities and the adherence to infection control guidelines has improved.
3.12. Inpatient vs. Outpatient Studies
Most of the studies were retrospective in nature and took into consideration only cases with positive urine cultures; they did not categorize inpatients or outpatients or specify which clinic was visited. Some studies focused only on the patients who visited EDs, as mentioned in Section 3.5 [2,3,32,33]. However, some studies focused only on inpatients and mostly on CAUTIs; as mentioned in Section 3.11, they did not report causative pathogens or their antimicrobial resistance patterns [31,45,48,56,57,61,62]. Throughout this review, E.coli was found to be the most common uropathogen, except in some studies that reported inpatient data where Klebsiella spp. was the main causative pathogen [35,54,66]. There was a higher MDR rate among inpatient samples [54]. Additionally, when the hospital stay was prolonged (>8 days), the risk for acquiring a UTI was significantly higher [45]. Two studies specifically reported on outpatients from nephrology clinics, and E.coli was reported as the main causative pathogen, but neither of those two studies reported the antimicrobial resistance pattern for those uropathogens [14,50].
4. Discussion
There were 58 publications published in KSA about UTIs, UTI prevalence, and the main UTI pathogens. UTIs were much more common among women than men. However, the prevalence was approximately equal between the sexes among inpatient CAUTI cases and among children under the age of 5 years [4,63,75]. Among the reasons why UTIs are more frequent among women is the anatomical structure of their urogenital system. Children under 5 years have a similar infection rate for hygiene reasons, but, as they mature, they tend to become more aware of hygiene and eventually decrease the risk. Another factor in the incidence of UTI among children is circumcision as uncircumcised boys tend to have a higher prevalence of UTI [9,21].
In our review, the Enterobacteriaceae family was the main cause for UTI. E. coli and Klebsiella spp. were the most common isolated organisms, and in some studies, they were the only reported organisms. They tended to have a strong antimicrobial resistance pattern; those isolated from inpatients were more likely to present with MDR and a high ESBL production rate [51,64,76]. Another major problem contributing to the high resistance rate seems to be inappropriate antibiotic prescription. There was a high prevalence of inappropriate antibiotic prescriptions, especially in emergency rooms, which affected mostly children and older adult patients. This might be among the reasons why patients with UTI are admitted to ED very frequently. It was also reported that there was a high readmission rate among patients with inappropriate antimicrobial prescriptions. This issue was addressed only by two papers, and they revealed major inappropriateness and recommended more focus be given to this issue [2,32].
Hospital-acquired UTIs, especially CAUTIs, are a major challenge, and investigation, the measures taken, and incentives given seem to have had a good effect because these infections have significantly decreased in the last two decades [56,66,77]. The CAUTI incidence was similar between males and females; this could be due to immunosuppression, catheter usage, and/or other factors related to nosocomial infections [56].
Women in general are at high risk for developing UTIs, and pregnancy tends to increase the risk. The presence of a UTI seems to be related to other conditions during pregnancy, such as anemia, hypertension and even premature birth [69,73]. A similar risk of UTI was found among patients with diabetes; infection tended to be associated with uncontrolled diabetes [14,31,46]. These associations might be due to immunosuppression that pregnant women and diabetic patients experience.
All the included studies had adequate sample sizes. All studies enrolled more than 100 cases; only five had a sample of less than 100, and a few had more than 1000 cases. Many of the papers assessed other medical conditions (e.g., pregnancy, cancer, diabetes, and intensive care unit) and their correlation with UTI prevalence.
Most of the papers were descriptive papers and did not have any significant statistical analysis. Moreover, apart from a few papers, most studies were retrospective, included only UTI-positive samples, and were not compared to a control group to assess the risk factors for developing a UTI, and resulted in a 100% prevalence rate [12,13,15,16,18,19,20,21,24,25,26,27,28,29,30,32,36,37,41,43,50,51,52,55,58,59,60,67]. Most of the publications were retrospective and lacked data on patients’ course of treatment and the prevalence of UTI reoccurrence and complications. Additionally, not all regions were equally represented. As mentioned above, some regions were continuously studied, while some others were represented in only one or two studies.
Antimicrobial resistance is growing at a concerning rate. A continuous, more extensive, and inclusive study is needed to understand the full antimicrobial sensitivity pattern both locally and nationally in KSA and to plan an updated treatment regimen.
Improper antibiotic prescription is another factor that leads to complicated UTI and an increased rate of antimicrobial resistance, but, unfortunately, only a few papers highlighted this issue. The nature of most studies was retrospective, and there was no patient follow-up until the end of the treatment and recovery from the infection. Thus, data were limited on the appropriateness of the treatments. Moreover, the data of prescribed empirical therapy and its appropriateness for the microbiology report were not reported. Future studies should focus on these matters.
This review paper was the outcome of a comprehensive search of multiple databases with specific keywords and careful examination of the titles and manuscripts of found publications. Therefore, it is unlikely that we missed any relevant English studies. However, any publication in Arabic only or nonpublished data on UTIs in KSA may have been missed. Additionally, some papers may have been excluded due to a lack of clearly specified samples/populations.
5. Conclusions
UTIs remain a major challenge for the healthcare system. Women and patients with underlying conditions tend to be at a higher risk, and E. coli remains the major causative pathogen. The current major concern is the rising antimicrobial resistance rate which requires rigorous scientific inquiry into antimicrobial resistance. Large epidemiological studies at the regional and national levels are needed to battle the rapid emergence of antimicrobial resistance. On the other hand, prospective and longitudinal studies are needed to assess the effectiveness of initially prescribed antibiotics. The data that can be obtained from these future studies will be valuable in developing new guidelines for the use of empiric therapies and controlling the increased rate of antimicrobial resistance.
Acknowledgments
We thank Erin Strotheide for her editorial contributions.
Author Contributions
Conceptualization, I.S. and M.A.A.; methodology, M.A.A.; software, I.S.; validation, M.A.A., A.M.H. and N.S.; formal analysis, N.S.; investigation, I.S.; resources, I.S.; data curation, I.S. and N.A.; writing—original draft preparation, I.S. and N.A.; writing—review and editing, I.S. and N.S.; visualization, I.S.; supervision, M.A.A. and N.S.; project administration, I.S. and M.A.A.; All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Data is available upon reasonable request to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Flores-Mireles A.L., Walker J.N., Caparon M., Hultgren S.J. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 2015;13:269–284. doi: 10.1038/nrmicro3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salam M., Al Anazi M., Al-Jeraisy M. Prevalence and predictors of antibiotic prescription errors in an emergency department, Central Saudi Arabia. Drug Healthc. Patient Saf. 2015;7:103–111. doi: 10.2147/DHPS.S83770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alrashid S., Ashoor R., Alruhaimi S., Hamed A., Alzahrani S., Al Sayyari A. Urinary Tract Infection as the Diagnosis for Admission Through the Emergency Department: Its Prevalence, Seasonality, Diagnostic Methods, and Diagnostic Decisions. Cureus. 2022;14:e27808. doi: 10.7759/cureus.27808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.E Silva A.C.S., Oliveira E.A. Update on the approach of urinary tract infection in childhood. J. Pediatr. 2015;91:S2–S10. doi: 10.1016/j.jped.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Goossens H., Ferech M., Vander Stichele R., Elseviers M., ESAC Project Group Outpatient antibiotic use in Europe and association with resistance: A cross-national database study. Lancet. 2005;365:579–587. doi: 10.1016/S0140-6736(05)17907-0. [DOI] [PubMed] [Google Scholar]
- 6.The World Health Report 2007: A Safer Future: Global Public Health Security in the 21st Century. [(accessed on 29 November 2022)]. Available online: https://apps.who.int/iris/handle/10665/43713?locale-attribute=ar&order=desc&scope=&sort_by=score&rpp=10&query=Theworldhealthreport2007:Asaferfuture:Globalpublichealthsecurityinthe21stcentury&search-result=true.
- 7.Warren J.W., Abrutyn E., Hebel J.R., Johnson J.R., Schaeffer A.J., Stamm W.E. Guidelines for Antimicrobial Treatment of Uncomplicated Acute Bacterial Cystitis and Acute Pyelonephritis in Women. Clin. Infect. Dis. 1999;29:745–758. doi: 10.1086/520427. [DOI] [PubMed] [Google Scholar]
- 8.European Centre for Disease Prevention and Control. European Medicines Agency . The Bacterial Challenge. Time to React: A Call to Narrow the Gap between Multidrug-RESISTANT bacteria in the EU and the Development of New Antibacterial Agents. European Union Publications; Luxembourg: 2009. [Google Scholar]
- 9.Kumar V., George A., Viswanathakumar M. Study of clinical profile and risk factors associated with febrile urinary tract infection in preschool children. Int. J. Contemp. Pediatr. 2016;3:243–246. doi: 10.18203/2349-3291.IJCP20160168. [DOI] [Google Scholar]
- 10.Lee S.J. Clinical Guideline for Childhood Urinary Tract Infection (Second Revision) Child. Kidney Dis. 2015;19:56–64. doi: 10.3339/chikd.2015.19.2.56. [DOI] [Google Scholar]
- 11.Antimicrobial Stewardship Subcommittee of the National Antimicrobial Resistance Committee and the General Administration of Pharmaceutical Care at Ministry of Health, Saudi Arabia National Antimicrobial Therapy Guidelines for Community and Hospital Acquired Infections in Adults. [(accessed on 29 November 2022)];2018 Available online: https://www.moh.gov.sa/en/CCC/healthp/regulations/Documents/National%20Antimicrobial%20%20Guidelines.pdf.
- 12.Alamri A., Hassan B., Hamid M.E. Susceptibility of hospital-acquired uropathogens to first-line antimicrobial agents at a tertiary health-care hospital, Saudi Arabia. Urol. Ann. 2021;13:166. doi: 10.4103/UA.UA_109_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balkhi B., Mansy W., Alghadeer S., Alnuaim A., AlShehri A., Somily A. Antimicrobial susceptibility of microorganisms causing Urinary Tract Infections in Saudi Arabia. J. Infect. Dev. Ctries. 2018;12:220–227. doi: 10.3855/jidc.9517. [DOI] [PubMed] [Google Scholar]
- 14.Almaiman L., Allemailem K.S., El-Kady A.M., Alrasheed M., Almatroudi A., Alekezem F.S., Elrasheedy A., Al-Megrin W.A., Alobaid H.M., Elshabrawy H.A. Prevalence and Significance of Pyuria in Chronic Kidney Disease Patients in Saudi Arabia. J. Pers. Med. 2021;11:831. doi: 10.3390/jpm11090831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hameed T., Al Nafeesah A., Chishti S., Al Shaalan M., Al Fakeeh K. Community-acquired urinary tract infections in children: Resistance patterns of uropathogens in a tertiary care center in Saudi Arabia. Int. J. Pediatr. Adolesc. Med. 2019;6:51–54. doi: 10.1016/j.ijpam.2019.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kader A.A., Angamuthu K. Extended-spectrum beta-lactamases in urinary isolates of Escherichia coli, Klebsiella pneumoniae and other gram-negative bacteria in a hospital in Eastern Province, Saudi Arabia. Saudi Med. J. 2005;26:956–959. [PubMed] [Google Scholar]
- 17.Alzahrani M.A., Sadoma H.H.M., Mathew S., Alghamdi S., Malik J.A., Anwar S. Retrospective Analysis of Antimicrobial Susceptibility of Uropathogens Isolated from Pediatric Patients in Tertiary Hospital at Al-Baha Region, Saudi Arabia. Healthcare. 2021;9:1564. doi: 10.3390/healthcare9111564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alasmary M.Y. Antimicrobial Resistance Patterns and ESBL of Uropathogens Isolated from Adult Females in Najran Region of Saudi Arabia. Clin. Pract. 2021;11:650–658. doi: 10.3390/clinpract11030080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bamshmous Y.S., Alwagdani S.M., Albarakati M.M., Alkouwait M.J., AlThwebi S.M. Infection with Gram-negative Bacteria among Children at a Tertiary Hospital in Jeddah, Saudi Arabia. Saudi J. Kidney Dis. Transplant. 2021;32:1593. doi: 10.4103/1319-2442.352420. [DOI] [PubMed] [Google Scholar]
- 20.Garout W.A., Kurdi H.S., Shilli A.H., Kari J.A. Urinary tract infection in children younger than 5 years. Saudi Med. J. 2015;36:497–501. doi: 10.15537/smj.2015.4.10770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alshamsan L., Al Harbi A., Fakeeh K., Al Banyan E. The value of renal ultrasound in children with a first episode of urinary tract infection. Ann. Saudi Med. 2009;29:46–49. doi: 10.4103/0256-4947.51817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al-Ibrahim A.A., Girdharilal R.D., Akhter M., Jalal C., Alghamdy A.H., Ghazal Y.K. Urinary Tract Infection and Vesicouretral Reflux in Saudi Children. Saudi J. Kidney Dis. Transpl. 2002;13:24–28. [PubMed] [Google Scholar]
- 23.Al-Harthi A.A., Al-Fifi S.H. Antibiotic resistance pattern and empirical therapy for urinary tract infections in children. Saudi Med. J. 2008;29:854–858. [PubMed] [Google Scholar]
- 24.al Johani S.M., Akhter J., Balkhy H., El-Saed A., Younan M., Memish Z. Prevalence of antimicrobial resistance among gram-negative isolates in an adult intensive care unit at a tertiary care center in Saudi Arabia. Ann. Saudi Med. 2010;30:364. doi: 10.4103/0256-4947.67073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akbar D.H. Urinary tract infection. Diabetics and non-diabetic patients—PubMed. Saudi Med. J. 2001;22:326–329. [PubMed] [Google Scholar]
- 26.Kader A.A., Nasimuzzaman M. Antimicrobial Resistance Patterns of Gram-Negative Bacteria Isolated from Urine Cultures in Almana General Hospital. Ann. Saudi Med. 2001;21:110–112. doi: 10.5144/0256-4947.2001.110. [DOI] [PubMed] [Google Scholar]
- 27.Alamri A., Hamid E.M., Abid M., Alwahhabi A.M., Alqahtani K.M., Alqarni M.S., Abomughaid M. Trend analysis of bacterial uropathogens and their susceptibility pattern: A 4-year (2013–2016) study from Aseer region, Saudi Arabia. Urol. Ann. 2018;10:41–46. doi: 10.4103/UA.UA_68_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alotaibi B.S., Tantry B.A., Farhana A., Alammar M.A., Shah N.N., Mohammed A.H., Wani F., Bandy A. Resistance Pattern in Mostly Gram-negative Bacteria Causing Urinary Tract Infections. Infect. Disord. Drug Targets. 2023;23:56–64. doi: 10.2174/1871526522666220928115043. [DOI] [PubMed] [Google Scholar]
- 29.Alsohaim S.I.A., Bawadikji A.A., Elkalmi R., Mahmud M.I.A.D.M., Hassali M.A. Relationship Between Antimicrobial Prescribing and Antimicrobial Resistance Among UTI Patients at Buraidah Central Hospital, Saudi Arabia. J. Pharm. Bioallied Sci. 2019;11:162. doi: 10.4103/JPBS.JPBS_217_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahmed S.S., Shariq A., Alsalloom A.A., Babikir I.H., Alhomoud B.N. Uropathogens and their antimicrobial resistance patterns: Relationship with urinary tract infections. Int. J. Health Sci. 2019;13:48. [PMC free article] [PubMed] [Google Scholar]
- 31.Al-Helali N.S., Al-Asmary S.M., Abdel-Fattah M.M., Al-Jabban T.M., Al-Bamri A.-L.M. Epidemiologic study of nosocomial urinary tract infections in Saudi military hospitals. Infect. Control Hosp. Epidemiol. 2004;25:1004–1007. doi: 10.1086/502336. [DOI] [PubMed] [Google Scholar]
- 32.Alanazi M.Q. An evaluation of community-acquired urinary tract infection and appropriateness of treatment in an emergency department in Saudi Arabia. Ther. Clin. Risk Manag. 2018;14:2363. doi: 10.2147/TCRM.S178855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alanazi M.Q., Alqahtani F.Y., Aleanizy F.S. An evaluation of E. coli in urinary tract infection in emergency department at KAMC in Riyadh, Saudi Arabia: Retrospective study. Ann. Clin. Microbiol. Antimicrob. 2018;17:3. doi: 10.1186/s12941-018-0255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sirkhazi M., Sarriff A., Aziz N.A., Almana F., Arafat O., Shorman M. Bacterial Spectrum, Isolation Sites and Susceptibility Patterns of Pathogens in Adult Febrile Neutropenic Cancer Patients at a Specialist Hospital in Saudi Arabia. World J. Oncol. 2014;5:196. doi: 10.14740/wjon850w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alghamdi S. Microbiological profile and antibiotic vulnerability of bacterial isolates from cancer patients. Cell. Mol. Biol. 2021;67:190–194. doi: 10.14715/cmb/2021.67.3.30. [DOI] [PubMed] [Google Scholar]
- 36.Bazaid A.S., Saeed A., Alrashidi A., Alrashidi A., Alshaghdali K., Hammam A.S., Alreshidi T., Alshammary M., Alarfaj A., Thallab R., et al. Antimicrobial Surveillance for Bacterial Uropathogens in Ha’il, Saudi Arabia: A Five-Year Multicenter Retrospective Study. Infect. Drug Resist. 2021;14:1455–1465. doi: 10.2147/IDR.S299846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alqasim A., Jaffal A.A., Alyousef A.A. Prevalence of multidrug resistance and extended-spectrum β -Lactamase carriage of clinical uropathogenic Escherichia coli isolates in Riyadh, Saudi Arabia. Int. J. Microbiol. 2018;2018:3026851. doi: 10.1155/2018/3026851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Al-Agamy M.H., Shibl A.M., Hafez M.M., Al-Ahdal M.N., Memish Z.A., Khubnani H. Molecular characteristics of extended-spectrum β-lactamase-producing Escherichia coli in Riyadh: Emergence of CTX-M-15-producing E. coli ST131. Ann. Clin. Microbiol. Antimicrob. 2014;13:4. doi: 10.1186/1476-0711-13-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kader A.A., Kumar A., Dass S.M. Antimicrobial Resistance Patterns of Gram-Negative Bacteria Isolated from Urine Cultures at a General Hospital. Saudi J. Kidney Dis. Transplant. 2004;15:135. doi: 10.5144/0256-4947.2001.110. [DOI] [PubMed] [Google Scholar]
- 40.El-Bashier A.M. Bacteriuria, Incidence, Causative Microorganism, and Susceptibility Pattern at Qatif Central Hospital. Ann. Saudi Med. 1991;11:429–434. doi: 10.5144/0256-4947.1991.429. [DOI] [PubMed] [Google Scholar]
- 41.Kader A.A., Kumar A. Prevalence and antimicrobial susceptibility of extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae in a general hospital. Ann. Saudi Med. 2005;25:239. doi: 10.5144/0256-4947.2005.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Al-Asmary S.M., Al-Helali N.S., Abdel-Fattah M.M., Al-Jabban T.M., Al-Bamri A.-L.M. Infection Control Unit (Al-Bamri), Al-Hada Armed Forces Hospital, Taif, Kingdom of Saudi Arabia. 2004. [(accessed on 29 November 2022)]. Available online: www.smj.org.sa.
- 43.Eltahawy A.T., Khalaf R.M.F. Urinary Tract Infection at a University Hospital in Saudi Arabia: Incidence, Microbiology, and Antimicrobial Susceptibility. Ann. Saudi Med. 1988;8:261–266. doi: 10.5144/0256-4947.1988.261. [DOI] [Google Scholar]
- 44.Abduljabbar H., Moumena R.A., Mosli H.A., Khan A.S., Warda A. Urinary Tract Infection in Pregnancy. Ann. Saudi Med. 1991;11:322–324. doi: 10.5144/0256-4947.1991.322. [DOI] [PubMed] [Google Scholar]
- 45.Balkhy H.H., Cunningham G., Chew F.K., Francis C., Al Nakhli D.J., Almuneef M.A., Memish Z.A. Hospital- and community-acquired infections: A point prevalence and risk factors survey in a tertiary care center in Saudi Arabia. Int. J. Infect. Dis. 2006;10:326–333. doi: 10.1016/j.ijid.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 46.Al-Rubeaan K.A., Moharram O., Al-Naqeb D., Hassan A., Rafiullah M.R.M. Prevalence of urinary tract infection and risk factors among Saudi patients with diabetes. World J. Urol. 2012;31:573–578. doi: 10.1007/s00345-012-0934-x. [DOI] [PubMed] [Google Scholar]
- 47.Abdulmutalib D.A., Abato A.T., Mazi W., Senok A. P017: Reduction of catheter associated urinary tract infections following removal of unnecessary urinary catheters in a tertiary care hospital in Saudi Arabia. Antimicrob. Resist. Infect. Control. 2013;2:P17. doi: 10.1186/2047-2994-2-S1-P17. [DOI] [Google Scholar]
- 48.Al-Tawfiq J.A., Amalraj A., Memish Z.A. Reduction and surveillance of device-associated infections in adult intensive care units at a Saudi Arabian hospital, 2004–2011. Int. J. Infect. Dis. 2013;17:e1207–e1211. doi: 10.1016/j.ijid.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 49.Hossain M.A., Mohal S., Islam M.S., Yusuf M.A. Prevalence of Ciprofloxacin Resistance among Gram-Negative Bacilli Isolated From Urinary Tract Infection Specimens at a Specialist Hospital in Riyadh, Saudi Arabia. J. Sci. Found. 2013;11:11–16. doi: 10.3329/jsf.v11i1.19395. [DOI] [Google Scholar]
- 50.Alharthi A.A., Taha A.A., Edrees A.E., Elnawawy A.N., Abdelrahman A.H. Screening for urine abnormalities among preschool children in western Saudi Arabia. Saudi Med. J. 2014;35:1477. [PMC free article] [PubMed] [Google Scholar]
- 51.El-Kersh T.A., Marie M.A., Al-Sheikh Y.A., Al-Kahtani S.A. Prevalence and risk factors of community-acquired urinary tract infections due to ESBL-producing Gram negative bacteria in an Armed Forces Hospital in Sothern Saudi Arabia. Glob. Adv. Res. J. Med. Med. Sci. 2015;4:321–330. [Google Scholar]
- 52.Faidah H.S., Ashshi A.M., El-Ella G.A.A., Al-Ghamdi A.K., Mohamed A.M. Urinary Tract Infections among Pregnant Women in Makkah, Saudi Arabia. Biomed. Pharmacol. J. 2015;6:01–07. doi: 10.13005/bpj/376. [DOI] [Google Scholar]
- 53.Al Yousef S.A., Younis S., Eman F., Moussa H.S., Bayoumi F.S., Ali A.M. Clinical and Laboratory Profile of Urinary Tract Infections Associated with Extended Spectrum β-Lactamase Producing Escherichia coli and Klebsiella pneumoniae. Ann. Clin. Lab. Sci. 2016;46:393–400. [PubMed] [Google Scholar]
- 54.Kabbani M.S., Ismail S.R., Fatima A., Shafi R., Idris J.A., Mehmood A., Singh R.K., Elbarabry M., Hijazi O., Hussein M.A. Urinary tract infection in children after cardiac surgery: Incidence, causes, risk factors and outcomes in a single-center study. J. Infect. Public Health. 2016;9:600–610. doi: 10.1016/j.jiph.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 55.Al-Mijalli S.H. Bacterial Uropathogens in Urinary Tract Infection and Antibiotic Susceptibility Pattern in Riyadh Hospital, Saudi Arabia. Cell. Mol. Med. 2017;3:1. doi: 10.21767/2573-5365.100028. [DOI] [Google Scholar]
- 56.Al-Hameed F.M., Ahmed G.R., Alsaedi A.A., Bhutta M.J., Al-Hameed F.F., Alshamrani M.M. Applying preventive measures leading to significant reduction of catheter-associated urinary tract infections in adult intensive care unit. Saudi Med. J. 2018;39:97. doi: 10.15537/smj.2018.1.20999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gaid E., Assiri A., McNabb S., Banjar W. Device-associated nosocomial infection in general hospitals, Kingdom of Saudi Arabia, 2013–2016. J. Epidemiol. Glob. Health. 2017;7((Suppl. 1)):S35–S40. doi: 10.1016/j.jegh.2017.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.al Wutayd O., al Nafeesah A., Adam I., Babikir I.H. The antibiotic susceptibility patterns of uropathogens isolated in Qassim, Saudi Arabia. J. Infect. Dev. Ctries. 2018;12:946–952. doi: 10.3855/jidc.10553. [DOI] [PubMed] [Google Scholar]
- 59.Albalawi S., Albalawi B., al Shwameen M.O., Alharbi M. Bacterial Susceptibility to Antibiotics in Urinary Tract Infections in Children, KSAFH, Saudi Arabia, Tabuk. Egypt. J. Hosp. Med. 2018;73:6952–6954. doi: 10.21608/ejhm.2018.17209. [DOI] [Google Scholar]
- 60.Taher I., Almaeen A., Aljourfi H., Bohassan E., Helmy A., Elmasry E., Saleh B., Aljaber N. Surveillance of antibiotic resistance among uropathogens in Aljouf region northern Saudi Arabia. Iran. J. Microbiol. 2019;11:468. doi: 10.18502/ijm.v11i6.2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alshamrani M.M., El-Saed A., Alsaedi A., El Gammal A., Al Nasser W., Nazeer S., Balkhy H.H. Burden of healthcare-associated infections at six tertiary-care hospitals in Saudi Arabia: A point prevalence survey. Infect. Control. Hosp. Epidemiol. 2019;40:355–357. doi: 10.1017/ice.2018.338. [DOI] [PubMed] [Google Scholar]
- 62.Ahmed N.J., Haseeb A., Elazab E.M., Kheir H.M., Hassali A.A., Khan A.H. Incidence of Healthcare-Associated Infections (HAIs) and the adherence to the HAIs’ prevention strategies in a military hospital in Alkharj. Saudi Pharm. J. 2021;29:1112–1119. doi: 10.1016/j.jsps.2021.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alrasheedy M., Abousada H.J., Abdulhaq M.M., Alsayed R.A., Alghamdi K.A., Alghamdi F.D., Al Muaibid A.F., Ajjaj R.G., Almohammadi S.S., Almohammadi S.S., et al. Prevalence of urinary tract infection in children in the kingdom of Saudi Arabia. Arch. Ital. Urol. Androl. 2021;93:206–210. doi: 10.4081/aiua.2021.2.206. [DOI] [PubMed] [Google Scholar]
- 64.Aabed K., Moubayed N., Alzahrani S. Antimicrobial resistance patterns among different Escherichia coli isolates in the Kingdom of Saudi Arabia. Saudi J. Biol. Sci. 2021;28:3776. doi: 10.1016/j.sjbs.2021.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Al-Mijalli S.H.S., Shami A.Y., Al-Salem R.A., Alnafisi N.M. Development of Diagnostic Capabilities for Complications of Bacterial Infection in Diabetic Patients. Rev. Diabet.Stud. 2022;18:135–139. doi: 10.1900/RDS.2022.18.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Saleem M., Khaja A.S.S., Hossain A., Alenazi F., Said K.B., Moursi S.A., Almalaq H.A., Mohamed H., Rakha E., Mishra S.K. Catheter-Associated Urinary Tract Infection in Intensive Care Unit Patients at a Tertiary Care Hospital, Hail, Kingdom of Saudi Arabia. Diagnostics. 2022;12:1695. doi: 10.3390/diagnostics12071695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aldecoa Y.S., Alanazi A., Saleh G.B., Alshanbari N., Humayun T., Alsheddi F., El-Saed A., Alqahtani M., Alanazi K.H. Rates of urinary catheter-associated urinary tract infection in Saudi MOH hospitals: A 2-year multi-centre study. Int. J. Infect. Control. 2022;18:21703. doi: 10.3396/ijic.v18.21703. [DOI] [Google Scholar]
- 68.Abalkhail A., AlYami A.S., Alrashedi S.F., Almushayqih K.M., Alslamah T., Alsalamah Y.A., Elbehiry A. The Prevalence of Multidrug-Resistant Escherichia coli Producing ESBL among Male and Female Patients with Urinary Tract Infections in Riyadh Region, Saudi Arabia. Healthcare. 2022;10:1778. doi: 10.3390/healthcare10091778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gupta K., Hooton T.M., Naber K.G., Wullt B., Colgan R., Miller L.G., Moran G.J., Nicolle L.E., Raz R., Schaeffer A.J., et al. International Clinical Practice Guidelines for the Treatment of Acute Uncomplicated Cystitis and Pyelonephritis in Women: A 2010 Update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin. Infect. Dis. 2011;52:e103–e120. doi: 10.1093/cid/ciq257. [DOI] [PubMed] [Google Scholar]
- 70.Rowe T.A., Juthani-Mehta M. Urinary tract infection in older adults. Aging Health. 2013;9:519–528. doi: 10.2217/ahe.13.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shapiro D.J., Hicks L.A., Pavia A.T., Hersh A.L. Antibiotic prescribing for adults in ambulatory care in the USA, 2007–2009. J. Antimicrob. Chemother. 2014;69:234–240. doi: 10.1093/jac/dkt301. [DOI] [PubMed] [Google Scholar]
- 72.Martínez M.A., Inglada L., Ochoa C., Villagrasa J.R., The Spanish Study Group on Antibiotic Treatments Assessment of antibiotic prescription in acute urinary tract infections in adults. J. Infect. 2007;54:235–244. doi: 10.1016/j.jinf.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 73.Habak P.J., Griggs R.P., Jr. Urinary Tract Infection In Pregnancy. StatPearls Publishing; Tampa, FL, USA: 2022. [(accessed on 17 December 2022)]. Available online: https://www.ncbi.nlm.nih.gov/books/NBK537047/ [PubMed] [Google Scholar]
- 74.Al-Asmary S.M., Al-Helali N.S., Abdel-Fattah M.M. Nosocomial urinary tract infection Risk factors, rates and trends. Saudi Med. J. 2004;25:95–900. [PubMed] [Google Scholar]
- 75.Flores-Mireles A., Hreha T.N., Hunstad D.A. Pathophysiology, Treatment, and Prevention of Catheter-Associated Urinary Tract Infection. Top. Spinal Cord Inj. Rehabil. 2019;25:228–240. doi: 10.1310/sci2503-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kandeel A. Prevalence and risk factors of extended-spectrum β-lactamases producing Enterobacteriaceae in a general hospital in Saudi Arabia. J. Microbiol. Infect. Dis. 2014;4:50–54. doi: 10.5799/ahinjs.02.2014.02.0126. [DOI] [Google Scholar]
- 77.Obaid N.A. Preventive Measures and Management of Catheter-Associated Urinary Tract Infection in Adult Intensive Care Units in Saudi Arabia. J. Epidemiol. Glob. Health. 2021;11:164. doi: 10.2991/jegh.k.210418.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is available upon reasonable request to the corresponding author.