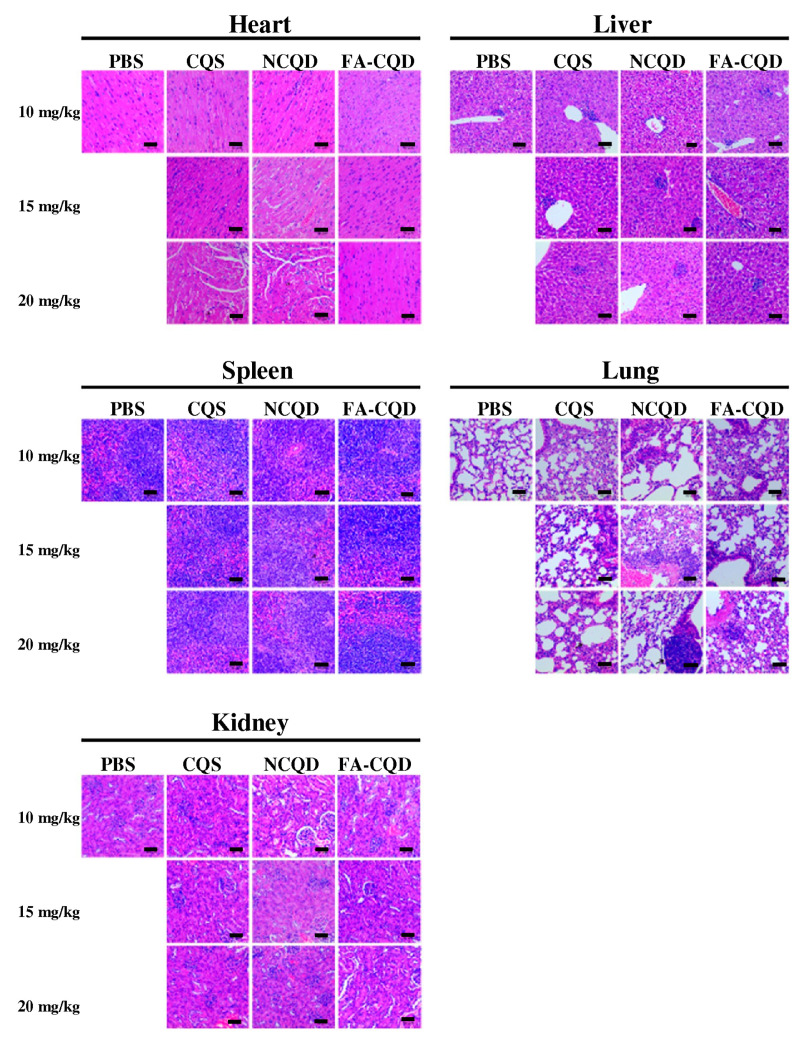
Figure 13.
Histological analysis of the CQD materials to evaluate toxicity. Histological study of the heart, liver, spleen, lung, and kidney of mice at 14 days after intravenous injection of the CQD materials at different concentrations (scale bar = 50 mm). Reprinted from Chinese Chemical Letters, 31, by Shu Zhang, Xibo Pei, Yiyuan Xue, Jingyuan Xiong, Jian Wang, Bio-safety assessment of carbon quantum dots, N-doped and folic acid modified carbon quantum dots: A systemic comparison, 1654, copyright 2020, with permission from Elsevier [296].