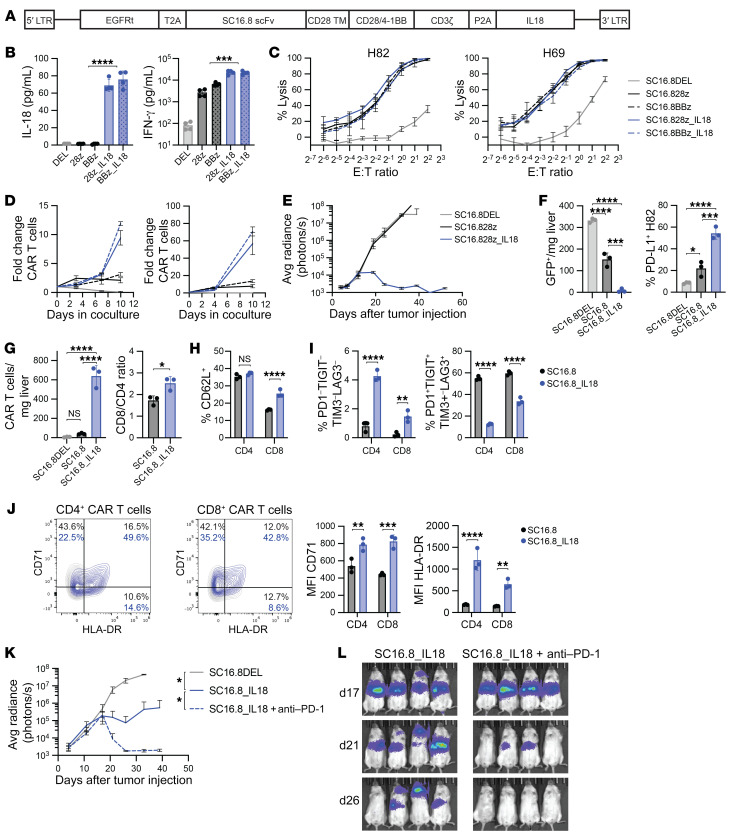
Figure 5. Human DLL3-targeting CAR T cells that secrete IL-18 are effective against SCLC and can be further potentiated by late-onset anti–PD-1 therapy.
(A) Schematic overview of the CAR design. EGFRt, truncated EGFR; scFv, single-chain variant fragment. (B) Levels of IL-18 and IFN-γ in cell culture supernatant of cocultures of the indicated CAR T cells and DLL3-expressing target cells. ***P < 0.001, ****P < 0.0001 (Student’s t test, n = 4). (C) Luciferase killing assay with H82 (DLL3+) and H69 (DLL3lo) SCLC cells. (D) CAR T cell proliferation in cocultures with H82 or H69 SCLC cells (E/T ratio of 1:5, n = 3, representative result from 3 independent experiments). (E) Growth curve of metastatic H82-SCLC tumors in mice that received 1 × 106 SC16.8 CAR T cells that did or did not secrete IL-18 (n = 4–5). (F–J) Livers of mice were subjected to flow cytometry analysis 9 days after CAR T cell treatment of mice with metastatic H82-SCLC tumors. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 (2-way ANOVA, n = 3). (F) Cell count of GFP+ H82 tumor cells (left) and PD-L1 expression on these GFP+ cells (right). (G) Cell count of EGFRt+ CAR T cells in the liver (left) and CD8/CD4 ratio of the CAR T cells (right). (H) Quantification of CD62L+ central memory CAR T cells. (I) Quantification of CD4+ and CD8+ CAR T cells that are quadruple negative (left) or quadruple positive (right) for the exhaustion markers PD-1, TIGIT, TIM3, and LAG3. (J) Representative flow plots and mean fluorescence intensity (MFI) of T cell activation markers CD71 and HLA-DR on CAR T cells. (K) Growth curve of metastatic H82-SCLC tumors in mice that received 0.3 × 106 CAR T cells alone or in combination with twice weekly 250 μg anti-human anti–PD-1 antibody, starting on day 18 (n = 4). (L) Bioluminescence imaging of same mice as in K, on days 17, 21, and 26 after tumor cell injection.