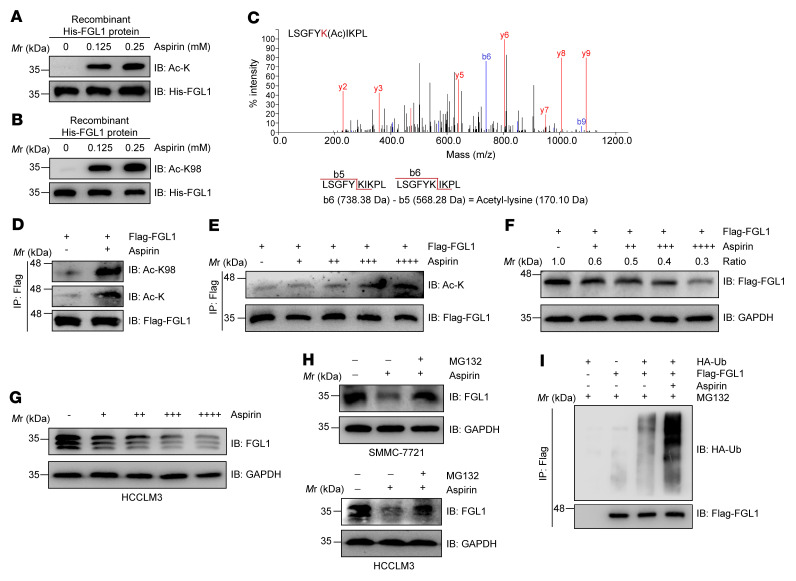
Figure 4. Aspirin acetylates FGL1 and promotes its degradation.
(A and B) Recombinant His-FGL1 protein was treated or not with aspirin (0.125 mM or 0.25 mM) for 30 minutes in vitro. FGL1 acetylation was assessed by IB using a pan–anti–acetyl-lysine antibody or site-specific anti–FGL1 acetylation antibody. (C) Mass spectrometric detection of Lys 98 acetylation derived from recombinant His-FGL1 protein treated or not with aspirin (0.25 mM) for 30 minutes in vitro. The y ion peaks are shown in red, and the b ion peaks are shown in blue. The mass difference between b6 and b5 is the mass of the acetyl-lysine residue. (D) IB analysis of FGL1 acetylation levels in HEK293T cells stably expressing Flag-FGL1 and treated or not with aspirin (0.25 mM, 12 h). Pan– or site-specific anti–FGL1 acetylation antibodies were used as indicated. (E) IB analysis of FGL1 acetylation levels in HEK293T cells stably expressing Flag-FGL1 and treated with increasing concentrations of aspirin (+, 0.0625 mM; ++, 0.125 mM; +++, 0.25 mM; ++++, 0.5 mM) for 12 hours. (F) IB analysis of FGL1 protein levels in HEK293T cells stably expressing Flag-FGL1 and treated with increasing concentrations of aspirin (+, 0.0625 mM; ++, 0.125mM; +++, 0.25mM; ++++, 0.5 mM) for 24 hours. (G) IB analysis of endogenous FGL1 protein levels in HCCLM3 cells treated with increasing concentrations of aspirin (+, 0.0625 mM; ++, 0.125mM; +++, 0.25mM; ++++, 0.5mM) for 24 hours. (H) IB analysis of endogenous FGL1 protein levels in SMMC-7721 and HCCLM3 cells treated with aspirin (0.25 mM, 24 h) in the presence or absence of MG132 (10 μM, 6 h). (I) IB analysis of FGL1 ubiquitination levels in HEK293T cells stably expressing Flag-FGL1 and treated with 0.25 mM aspirin for 12 hours.