Abstract
Lactoferrin is an iron-binding glycoprotein present in most human exocrine fluids, particularly breast milk. Lactoferrin is also released from neutrophil granules, and its concentration increases rapidly at the site of inflammation. Immune cells of both the innate and the adaptive immune system express receptors for lactoferrin to modulate their functions in response to it. On the basis of these interactions, lactoferrin plays many roles in host defense, ranging from augmenting or calming inflammatory pathways to direct killing of pathogens. Complex biological activities of lactoferrin are determined by its ability to sequester iron and by its highly basic N-terminus, via which lactoferrin binds to a plethora of negatively charged surfaces of microorganisms and viruses, as well as to mammalian cells, both normal and cancerous. Proteolytic cleavage of lactoferrin in the digestive tract generates smaller peptides, such as N-terminally derived lactoferricin. Lactoferricin shares some of the properties of lactoferrin, but also exhibits unique characteristics and functions. In this review, we discuss the structure, functions, and potential therapeutic uses of lactoferrin, lactoferricin, and other lactoferrin-derived bioactive peptides in treating various infections and inflammatory conditions. Furthermore, we summarize clinical trials examining the effect of lactoferrin supplementation in disease treatment, with a special focus on its potential use in treating COVID-19.
Keywords: lactoferrin, lactoferricin, lactoferrampin, antimicrobial peptides, innate immunity, immunomodulation, inflammatory disease, COVID-19
1. Introduction
Breast milk acts not only as the first nutrition for infants but also as the first barrier against infections after birth. However, milk also offers health benefits beyond infancy. Casein-free whey protein fractions of milk contain cytokines, growth factors, antibodies, complement components, and various antimicrobial proteins, such as lactoferrin (LF) [1,2,3], the subject of this review.
The human glycoprotein LF (initially named lactotransferrin), a member of the transferrin family, is present not only in human milk, but also in exocrine secretions and body fluids (e.g., tears, saliva, and urine), in secondary granules of neutrophils, and on mucosal surfaces in the respiratory, urinary, reproductive, and intestinal tracts [4,5,6].
LF was discovered over 80 years ago [7]; since then, numerous studies have documented its activities and mechanisms of action in host defense. LF is particularly abundant in colostrum, the first form of breast milk produced after childbirth, which is why LF earned its nickname “newborns’ guardian”. By breastfeeding, infants receive among others the benefits of LF, which helps to protect them from infections until their own immune system fully develops [8].
LF found in the milk of cows, goats, sheep, buffaloes, and camels is commonly used as a nutritional supplement for older children and adults [9,10]. However, commercially purified LF produced on a large scale from animal milk is not only sold as a food supplement but also used in the pharmaceutical industry [4].
Upon ingestion, nutritional LF is proteolytically cleaved releasing natural bioactive peptides, such as lactoferricin (LFC), that not only retain some of the biological activities of LF but may also provide additional benefits [11].
Generally, LF is considered to be a multifunctional protein that plays a significant role in the immune system [12,13]. Its functions are diverse, including antibacterial, antifungal, antiviral, antiparasitic, antioxidant, antitumor, anti-inflammatory, and immunomodulatory activities [14]. These protective functions primarily depend either on the ability of LF to sequester iron ions or on the binding capacity of the positively charged N-terminal region, from which the natural peptide LFC is derived [14,15].
In neonatal medicine, LF and its derived natural or synthetic peptides are considered to be some of the most valuable and best-researched compounds [16], but they are also often used as supplements in multiple clinical trials for diarrheic, respiratory, inflammatory, dermal, or malignant diseases [17]. Recently, due to their antiviral potential, LF and its derivatives have also gained intensive attention as potential therapeutic tools against Coronavirus disease 2019 (COVID-19) [18].
2. Lactoferrin (LF)
2.1. Structure of LF
LF is a non-heme iron-binding glycoprotein that belongs to the transferrin family. All proteins from the transferrin family share a similar three-dimensional structure. Human LF is made of about 700 amino acids with a molecular weight of approximately 80 kDa (Figure 1). LFs from different species exhibit high homology [5,9,14,15]. A single polypeptide chain of LF is folded into two lobes (N and C) with 33–41% mutual homology [19], which probably originated from gene duplication [6,14]. LF has a less flexible structure than transferrin, with a relatively rigid linker formed by a three-turn helix, which connects the two lobes [20,21,22]. This linker may mediate a cooperative interaction between the LF lobes, contributing to its capacity to bind iron even at low pH [23]. The lobes are composed of α-helices and β-sheets assembled into two domains (N1 and N2 or C1 and C2 for the N- or C-lobe, respectively), with a metal-binding cleft in the middle. Each lobe, via its two tyrosines, one aspartate, and one histidine, can reversibly bind a single ferric (Fe3+) ion. The cleft can accommodate also other metal ions including ferrous iron (Fe2+), copper (Cu2+), zinc (Zn2+), manganese (Mn2+), aluminum (Al3+), or cerium (Ce4+) ions, implying that LF could also play a role in the homeostasis of these microelements [5,24]. However, the biological significance of binding of metals other than iron has not been investigated. Metal ions are bound in synergy with a carbonate ion CO32−, which stabilizes them in the cleft through two oxygen atoms [5,20,21,24]. LF binds ferric iron with an extremely high affinity (Kd ~10−20 to 10−22 mol/L); affinities to other metal ions are markedly lower, and metal ion binding is accompanied by a conformational change within the protein structure [5,14,21,25]. Each lobe of LF can be either in the “closed” iron-bound state or in the “open” iron-free state [5,14]. Holo-LF with two bound iron cations displays a stable and conformationally rigid structure with iron ions difficult to remove. On the contrary, iron-free apo-LF is more flexible and prone to easier thermal denaturation and proteolysis. The release of iron depends on destabilization of the closed holo-form and is accompanied by a conformational change—domains in lobes swing away from each other to open the metal-binding clefts [5,25]. This is possible by lowering the pH, e.g., in the acidic compartments of cells (endosomes), leading to protonation of the carbonate ion, which results in weakening of the iron coordination to the point where it no longer holds the two domains together. Several available apo-LF X-ray structures display the closed configuration of the C-lobe, implying that open-close dynamics in solution might facilitate iron binding [5,14,22,25,26]. Furthermore, it has been observed that iron binding regulates human LF reactivity to glycan-binding proteins, suggesting that conformational changes accompanying iron chelation could be involved in the function of the molecule [27].
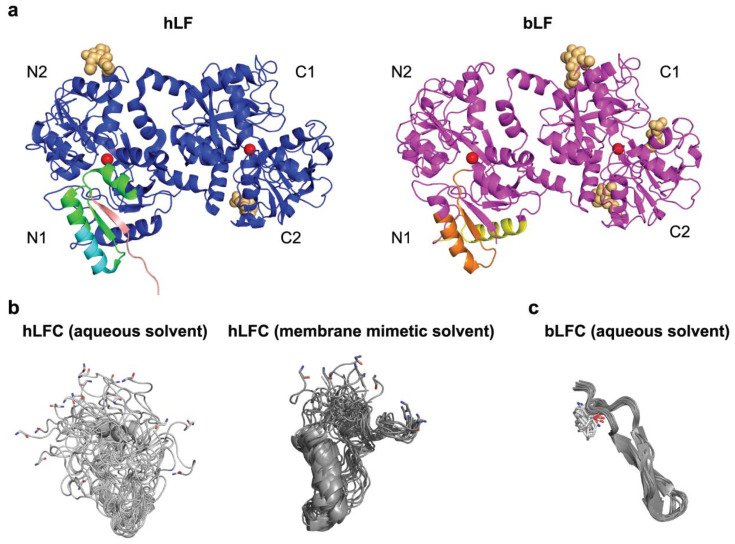
Figure 1.
Domain architecture of human (hLF) and bovine (bLF) lactoferrin and the localization of the bioactive peptides within. (a) X-ray structures of holo-hLF (PDB ID 2BJJ, blue cartoon) and holo-bLF (PDB ID 1BLF, magenta cartoon). The positions of individual domains in the N- and C-lobes are indicated. hLFC (residues 1–49) is highlighted in different colors (green, salmon, and cyan) in the N1 domain of hLF. In particular, stretches of hLFC carrying a pronounced antimicrobial activity, often studied in the form of synthetic peptides (hLFC (residues 1–11) and hLFC (residues 21–31)), are shown in salmon and cyan, respectively. bLFC (residues 17–41) and bovine lactoferrampin (bLFA) (residues 265–284) are shown in orange and yellow, respectively, in the N1 domain of bLF. Iron ions bound to bLF and hLF are shown as red spheres. Note the differences in the glycosylation pattern of both proteins (asparagine and the first N-acetylglucosamine sugar residue are shown as beige spheres). (b) Twenty NMR solution structures of hLFC in aqueous solution (PDB ID 1Z6W) and in a membrane mimetic solvent (PDB ID 1Z6V). hLFC adopts a mostly disordered coiled conformation in aqueous solution. The flexibility of this conformation facilitates an adaptive binding to negatively charged surfaces of pathogen proteins. In a membrane mimetic solvent, the central part of hLFC acquires an amphipathic helical conformation, similar to the one it adopts in intact LF, thereby acting to disrupt pathogen membranes. (c) Twenty NMR solution structures of bLFC in aqueous solution (PDB ID 1LFC, gray). In contrast to the αβ conformation that retains the same residues in intact bLF (as shown in panel (a)), the bLFC peptide has a strong tendency to form a distorted, amphipathic antiparallel β-sheet. The N-terminal glycine residues in (b) and the N-terminal phenylalanine residues in (c) are highlighted as sticks; with carbon, nitrogen, and oxygen atoms labeled in grey, blue, and red, respectively. All structures were generated using PyMOL software.
While LF and the prototype family member serum transferrin (TF) have the same global structure and use the same binding sites for binding iron, LF binds Fe3+ ions with 300-fold higher affinity than TF, and releases them at much lower pH values; LF was shown to retain iron to a pH as low as 3–4, compared to pH 5–6 for TF [5,23,26,28]. These properties set these two proteins functionally apart. Serum TF is primarily an iron transporter, whereas the main function of LF lies in efficient iron sequestration from the environment, via which LF contributes to innate immune defense and iron homeostasis (as explained in detail later) [6,14,22]. Further functional differences between LF and TF stem from the high number of basic (positively charged) residues contained in LF at its N-terminus (residues 1–7 and 13–30) and around the helix connecting the N- and C-lobes [5]. For this reason, LF is the most alkaline member of the transferrin family. The isoelectric point of LF is around 8.5–9.0 depending on the species, resulting in strong binding to many negatively charged extracellular, membrane-anchored, and intracellular molecules, such as DNA, lipopolysaccharide (LPS), or heparan sulfate proteoglycans (HSPGs) [5,6,14,18,29,30]. Lastly, LF is a glycoprotein with N-linked glycans, but the number and position of glycosylation sites vary between the mammalian species (Figure 1a) [5]. Human LF (hLF) has three, while bovine LF (bLF) has five potential N-glycosylation sites [5]. hLF contains core-fucosylated complex glycans (glycan antennas contain N-acetylglucosamine (GlcNAc), galactose, and sialic acid), whereas bLF contains both complex and high-mannose glycans (core GlcNAc dimer modified only with mannose residues) [31,32]. Glycosylation of both hLF and bLF found in milk exhibits dynamic changes over the course of lactation [32,33,34]. N-glycans do not influence LF binding of iron or LPS, but they protect it against tryptic proteolysis [5,35] and act as soluble decoy receptors for invasive pathogens that cause infections by binding to cells via their surface lectins, e.g., food-borne Salmonella typhimurium or Listeria monocytogenes [33,36]. LF glycosylation in multiple species was recently comprehensively reviewed by Zlatina and Galuska [31].
2.2. Tissue Distribution of LF
In situ hybridization and immunohistochemical analyses have revealed that LF is expressed during specific stages of murine embryogenesis. Its expression begins as early as the 2–4 cell fertilized embryo stage, continues until the blastocyst stage, and then becomes almost undetectable in the hatched blastocyst. This suggests that LF plays a role in preimplantation development. LF expression re-emerges later in gestation, specifically in neutrophils of the fetal liver and in epithelial cells of the respiratory and digestive systems [37]. There are no comprehensive reports on LF expression during human embryonic development yet. Only one report showed a positive correlation of LF concentrations in the follicular fluid with fertilization rate and embryo quality of in vitro fertilized patients, supporting the role of LF in the reproductive process in humans [38].
In adult humans, LF is secreted into mucosal fluids by glandular epithelial cells. It is constitutively present at the mucosal surface and regulated by various hormones and transcription factors in a tissue-specific manner [39,40]. Thus, LF is found in saliva, tears, semen, vaginal fluids, gastrointestinal fluids, nasal and bronchial secretions, and sweat [9,41,42]. However, the most enriched source of LF is breast milk with an approximate concentration of 2.6 mg/mL in human milk, 0.09 mg/mL in bovine milk, and the highest concentration in human colostrum with 5.3 ± 1.9 mg/mL [11,43,44,45,46].
Granulocyte colony-stimulating factor (G-CSF) induces expression of LF in secondary granules of neutrophils [47], where LF represents one of the major proteins with levels ranging from 3 to 15 µg/106 neutrophils [48,49]. LF released from neutrophils occurs also in plasma, albeit at low concentration (0.2–1.5 µg/mL) [49]. Neutrophil-derived LF has also been detected in feces, where the concentration significantly rises during inflammation, e.g., as a response to pathogenic bacteria and in inflammatory bowel diseases (IBDs), such as ulcerative colitis and Crohn’s disease [50]. In this respect, fecal LF has been proposed as a specific and noninvasive marker to differentiate between IBD and noninflammatory irritable bowel syndrome [50,51]. Moreover, LF can be released from microglial cells, the resident macrophages in the brain [52]. Additionally, it was suggested that LF production might be triggered in some cell types by apoptosis [53].
2.3. Receptors of LF
LF performs various functions by binding to a wide range of receptors on target cells (Table 1), resulting in a vast array of cellular responses [6,54]. For instance, via binding the low-density lipoprotein receptor-related protein-1/alpha 2-macroglobulin receptor (LRP-1, CD91) on fibroblasts, LF promotes a phenomenon called collagen gel contraction, which is essentially the ability of fibroblasts to reorganize the surrounding 3D collagen matrix into a more dense and compact structure during wound healing [55]. By binding to LRP-1 on fibroblasts, LF also blocks LRP-1-dependent stimulation of cholesteryl ester synthesis elicited by β-very-low-density lipoprotein complexes [56]. Lastly, via LRP-1, LF has been shown to stimulate osteoblast proliferation [57]. Via C–X–C motif chemokine receptor 4 (CXCR4, CD184), LF induces the activation of AKT signaling in human epithelial cells [58]. In the intestine, LF and its derivatives have been shown to bind the glycan-binding lectin intelectin-1 (also known as omentin-1) for resorption of ingested LF and direct immunomodulation in the gastrointestinal tract [59,60]. LF was found to bind with high affinity to the soluble form of the LPS coreceptor CD14 [61]; via LPS, it was also shown to bind to Toll-like receptor 4 (TLR4) [62]. Moreover, interactions with TLR2 and endosomal TLR9 in macrophages and antagonism of their pattern recognition role have been reported, which resulted in LF-mediated suppression of the TLR2- and TLR9-mediated proinflammatory response against Epstein–Barr virus [63]. Furthermore, one of the C-type lectin receptors, termed dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN, CD209) was identified as an interaction partner of LF in dendritic cells (DCs) [64]. LF has also been reported to bind to proliferating cells, including immune cells [65]. In this case, the cell surface-expressed nucleolin was identified as the LF receptor. This interaction, however, does not lead to signaling; instead, the nucleolin–LF complex is internalized through vesicles of the recycling-degradation pathway [66]. Lastly, as mentioned earlier, LF via its basic N-terminus binds to HSPGs expressed on the surface of multiple cell types [11,18,67,68].
LF has also been shown to interact with several soluble molecules, such as ceruloplasmin, osteopontin, or plasminogen, in the blood or other biological fluids [6,69,70,71].
Table 1.
Cell surface receptors of LF and cell types in which they are expressed.
| LF Receptor | Expressed by | Selected References |
|---|---|---|
| LRP-1 (CD91) | Multiple cell types (fibroblasts, osteoblasts, myeloid cells) | [55,56,57,72] |
| CXCR4 (CD184) | Leukocytes, epithelial cells, platelets | [58,73] |
| Intelectin-1 (omentin-1) | Intestinal epithelial cells | [59,60] |
| CD14 | Monocytes, macrophages, neutrophils | [61,74] |
| TLR2 (CD282), TLR4 (CD284) | Myeloid cells, endothelial cells | [62,63,75] |
| DC-SIGN (CD209) | Myeloid cells (DCs, certain macrophage types) | [64,76,77] |
| Nucleolin | Proliferating cells | [66] |
| HSPGs | Broadly expressed on various cells and as extracellular matrix macromolecules | [11,67,68] |
3. LF-Derived Bioactive Natural and Synthetic Peptides
Ingested LF can be cleaved by pepsin in the stomach, but also by trypsin and chymotrypsin in the small intestine [78,79,80]. A previous study showed that hLF is largely digested by the digestive system of adults [78]. bLF might be more stable in the human digestive tract, since it was reported that more than 60% of both the apo- and the holo-forms of bLF resisted proteolytic digestion by pepsin in the stomach of study participants [81]. However, the two clinical trials performed by the same group are not directly comparable; the first trial assessed a digestion of 5 g of recombinant hLF in the stomach and the small intestine of ileostomy patients over 24 h, while the second assessed the digestion of 4.5 g of bLF in stomachs of probands with a nasogastric tube for up to 30 min [78,81]. In any case, the situation is different in newborns, whose gastrointestinal tract is not yet fully developed and unable to extensively digest LF present in human or cow milk, since it was shown that both hLF and bLF recovered from feces of infants were only partially hydrolyzed and also retained the iron-binding capacity [8,82]. Regardless of the outcomes of these studies, the digestion results in the generation of antimicrobial peptides, namely, LFCs (Figure 1).
3.1. Lactoferricin (LFC) and Derived Peptides
The bioactive peptides can be released from LF by pepsin cleavage in the acidic environment of the stomach. In the literature, they are sometimes collectively termed LFCs, which is ambiguous and may be confusing. In this work, we reserve the term LFC for the longest peptide released after pepsin cleavage, whereas shorter peptides derived from LFC are specified by amino-acid numbering according to the LF sequence.
Both bovine LFC (bLFC) and human LFC (hLFC) were first identified in 1992 by proteolytic digestion of bLF and hLF, respectively, with pepsin in vitro [83]. Later, bLFC was confirmed as a breakdown product of bLF in the human stomach [79]. Both peptides are derived from the highly basic N-terminal region of LF, are resistant to further pepsin cleavage, and preserve many activities of LF or are even more potent than the parental LF [83,84,85,86].
Due to the shared antimicrobial properties of LF and LFC, the activity of intact LF is commonly explained as a specific action of its N-terminus from which the LFC peptide is derived [84]. However, nuclear magnetic resonance spectroscopy studies have shown that, in contrast to intact LF, wherein the LFC-encompassing sequence adopts a βαβα motif [5], the conformation of the free LFC peptides is radically different (Figure 1) [84,87]. LFCs also lack the iron-binding capacity [11].
bLFC is a 25-residue peptide (corresponding to residues 17–41 of bLF) and folds in aqueous solution into an amphipathic hairpin with two antiparallel β-strands and one intramolecular disulfide bond [88]. hLFC is twice as long as bLFC, consisting of the N-terminal 49 residues (i.e., residues 1–49) of mature hLF in a single continuous chain stabilized by two intramolecular disulfide bonds. In contrast to bLFC, hLFC is mostly disordered in aqueous solution but it shows a propensity of an α-helix in a membrane mimetic solvent (Figure 1) [83,84,87]. hLFC bears nine positive charges at neutral pH; four of them are located as the tetra arginine sequence at positions 2–5 [83,84]. Both bLFC and hLFC are able to adopt an amphipathic conformation in membrane mimetic solvents with separated hydrophobic (represented by tryptophan residues) and positively charged hydrophilic (represented by arginine residues) surface regions, which is a specific feature of antimicrobial peptides. The amphipathic properties of LFCs, and antimicrobial peptides in general, enable an interaction with lipidic molecules, as well as with negatively charged surfaces of Gram-negative and Gram-positive bacteria, fungi, parasites, viruses, and tumor cells; through binding to negatively charged patterns, they exhibit antibacterial, antifungal, antiparasitic, antiviral, and antitumor activity [11,46,84].
The extremely basic character is a specific feature of LFCs, and both the high net positive charge and the position of the cationic residues appear to be important for the afore-mentioned activities [84]. A stabilized secondary structure is also important, as the absence of the disulfide bond resulting in a disruption of the cyclic form of both bLFC and hLFC impairs antibacterial and antiviral activity [89,90,91,92]. Differences in length and amino-acid composition of bLFC and hLFC, both mirrored in their secondary structures, result, to some extent, in different effectiveness; bLFC is considered the more potent one out of the two peptides [84,89,93].
Most research has examined the antimicrobial properties of bLFC and hLFC, as well as synthetic peptides derived from them. However, a limited number of studies have also investigated LFCs from other species such as pig, mouse, goat, and camel [89,92,94,95,96,97]. All these LFCs seem to be active, but bLFC beats them with its superior bactericidal activity [89].
3.2. Lactoferrampin (LFA)
Additional bioactive peptides derived from LF are termed LFAs. LFA was first identified from bLF and synthesized chemically in 2004 [98]. Like LFC, LFA is derived from the cationic N-lobe, specifically bLF residues 268–284, and it possesses antibacterial and antifungal properties [98]. In 2005, the same research group described a more potent and slightly longer peptide (comprising residues 265–284; Figure 1a, right) [99]. As many antimicrobial peptides, LFA also adopts an amphipathic α-helical conformation in membrane mimetic solvents, at least at the N-terminus, but remains relatively unstructured at the strongly positively charged C-terminus that is essential for its antimicrobial activity [99,100,101]. However, the corresponding region of hLF does not possess antimicrobial activity. It can, however, be modified in vitro by increasing the net positive charge near the C-terminus, thus gaining this potency [101].
Notably, a synthetic fusion peptide of bLFC and bLFA, termed LFchimera, has higher antibacterial activity than the individual bovine bioactive peptides, even against multidrug-resistant bacteria and against strains that normally form biofilms [102,103,104,105,106]. LFchimera was also shown to have a superior parasiticidal effect against the enteric parasites Giardia intestinalis and Entamoeba histolytica [107,108], as well as a profound anticancer effect [93].
4. LF and LFC in Host Defense
Although scientific advances have led to large-scale production and widespread distribution of vaccines, but also antiviral and antimicrobial drugs, viruses and microorganisms still remain one of the major threats worldwide. The ever-increasing number of reports of pathogen resistance, as well as the emergence and re-emergence of epidemics, force the medical and scientific community to constantly search for new molecules with protective potential. Antimicrobial proteins and peptides have proven to be a promising alternative. LF and its derivatives, via various features, such as iron sequestration, pathogen membrane disruption, peculiar binding properties, modulation of immune cell activation and function, and protease blockade, are crucial molecular players in host defense processes, against viruses and bacteria in particular.
4.1. LF in Iron Homeostasis
Initially, LF was thought to play a role in intestinal iron uptake, transport, and delivery in both neonates and adults. However, these theories were disproven in 2003 when research on LF-knockout mice revealed that they were viable, fertile, and did not exhibit any abnormalities in their serum iron levels [60]. Today, LF is recognized as a guardian of iron homeostasis, as it efficiently scavenges toxic free iron from the environment [22,109]. Free iron ions released from necrotic cells in infected and/or inflamed tissue have a central role in oxidative stress due to induction of free radicals, i.e., reactive oxygen species (ROS) and reactive nitrogen species (RNS) [13,22]. In this scenario, the iron-scavenging ability of apo-LF released from the secondary granules of neutrophils functions as an antioxidant, protecting tissues from unwanted free iron-induced formation of ROS and damage to cell membranes, proteins, lipids, and nucleic acids [22,110,111]. Similarly, such scavenging of iron deposits in the brains of Alzheimer and Parkinson disease patients may be maintained by LF released by activated microglia [52,112].
In addition, via iron sequestering, LF reduces iron availability for iron-dependent pathogens, which contributes to host protection against infections via so-called nutritional immunity [113,114]. Because of this, LF is considered an important component of the innate immune defense system [5,6,14].
Interestingly, the iron-regulatory role of LF can be exploited therapeutically; iron-loaded holo-LF has been shown to efficiently deliver iron into cancer cells and sensitize them to radiotherapy and ferroptosis (an iron-dependent regulated cell death) via increased ROS production [115].
4.2. LF and LFC in Immunomodulation
Neutrophils play a crucial role in the immune response. During inflammation, neutrophils are attracted to the site of injury or infection in large numbers, more than any other type of white blood cells. They play a crucial role in killing invading bacteria by phagocytosis or by releasing antimicrobial peptides at the site of infection [116]. Additionally, they release a vast amount of LF from their secondary granules [12,117], and it was observed that released LF acts as an alarmin to attract antigen-presenting cells (monocytes, macrophages, and DCs) to the inflamed site [118]. In this respect, LF-mediated recruitment of DCs is especially important, as this creates the link to the adaptive immune responses [117].
LF is released from neutrophil secondary granules predominantly in its iron-free form (apo-LF), and, by scavenging free iron, it contributes to bacterial killing via iron deprivation [113]. Additionally, LF normalizes the redox status within the tissue as outlined above, which is beneficial, because, even though ROS and RNS production is intimately linked to immune cell activation, their excessive and prolonged generation impairs the inflammatory responses of several immune cell types, including macrophages, T cells, and B cells, and can even lead to cell death [119,120].
The most prominent immunomodulatory effects of LF are attributed to its highly basic N-terminal region formed by the positively charged arginine and lysine residues [5,18,29,30]. Via this region, LF interacts with a broad spectrum of binding partners, e.g., LPS from cell walls of Gram-negative bacteria [121], lipoteichoic acid (LTA) from cell walls of Gram-positive bacteria [122], DNA, including unmethylated CpG islands found in microbial DNA [123,124], glycosaminoglycans [30], heparin [125], and other negatively charged molecules encompassed in both pathogens and host cells. Many of these LF ligands belong to the pathogen-associated molecular patterns (PAMPs) that are sensed by pattern recognition receptors (PRRs), such as Toll-like receptors (TLRs) on the surface of immune and certain nonimmune cells. Early detection of PAMPs is crucial for the initiation of innate immune responses, the ultimate goal of which is pathogen elimination. For example, free LPS, a very potent endotoxin, binds to the serum protein termed LPS-binding protein (LBP). The LPS–LBP complex is then transferred to CD14, which accelerates LPS recognition by the TLR4/myeloid differentiation-2 (MD-2) complex [126]. LPS-triggered homodimerization of the TLR4/MD-2/LPS complexes leads to the assembly of specific adaptors (MyD88—myeloid differentiation factor 88 and TRIF—Toll/interleukin-1 receptor domain containing adaptor inducing interferon-β). This triggers a signaling cascade resulting in activation of various transcription factors such as NF-κB to induce expression of cytokines, e.g., tumor necrosis factor-alpha (TNF-α), interleukin-1β (IL-1β), IL-6, and type I interferons (IFNs) [126]. TLR4-mediated signaling also induces ROS and RNS in myeloid cells [119] and promotes DC maturation, thereby linking innate and adaptive immunity [126].
LF antagonizes this meticulously organized cascade on several levels to prevent excessive LPS signaling via TLR4 and, hence, to exert anti-inflammatory effects. Firstly, LF competes with LBP for LPS binding, and it prevents the interaction of LPS with CD14 [127], which results in reduced production of proinflammatory cytokines from LPS-stimulated monocytes and macrophages [128,129,130]. Secondly, LF interacts with both free soluble CD14 (sCD14) and the sCD14–LPS complex, thereby preventing LPS-triggered activation of endothelial cells via TLR4 and upregulation of endothelial adhesion molecules [61]. Thirdly, LF treatment leads to downregulation of TLR4 expression on the macrophage surface [130].
On the other hand, LF has been also shown to promote macrophage activation via TLR4-dependent and TLR4-independent pathways in certain in vitro studies [62,75,131]. This could be in part related to its “contamination” by LPS, as LF is a very potent scavenger and carrier of this endotoxin [12,13,131,132]. Further experiments with LPS-depleted LF revealed that hLF is able to mildly activate the TLR4 pathway due to recognition of LF carbohydrate chains, probably complexed with CD14. However, when cells were simultaneously treated with LPS and LPS-depleted hLF, cytokine production was again dampened compared to treatment with LPS alone. This suggests that hLF may have a role as a moderate activator of the immune system, while it effectively neutralizes the strong proinflammatory actions of LPS [75]. Accordingly, LF administration is protective even against lethal challenge in experimental endotoxemia (intravenous or intraperitoneal LPS injection) or bacteremia (infection with live Escherichia coli) in rodents [133,134,135,136,137,138]. LF treatment substantially reduced the serum levels of LPS-induced cytokines TNF-α, IL-6, IL-10 and nitric oxide (NO) [134], and effectively counteracted LPS- and E. coli-induced intestinal injury that otherwise manifested as diarrhea [133,137,138]. Protective effects of LF against damage to the intestinal mucosa were attributed to its capacity to attenuate increased epithelial inflammation and permeability due to disruption of epithelial tight junctions caused by LPS [139,140,141].
Regarding other immunomodulatory functions of LF, it was observed that LF triggered maturation of monocyte-derived DCs (moDCs) through TLR2 and TLR4, which supported antigen-specific T cell responses in coculture experiments with efficient differentiation of naïve T cells toward the Th1 type [118,138,142]. LF-matured DCs displayed an enhanced release of the inflammatory chemokines CXCL-8 and CXCL-10 and dampened production of IL-6, IL-10, and CCL-20. This effect was not due to LPS contamination of the recombinant hLF used in these experiments [142]. Moreover, it was recently observed that, in the presence of ssRNA, bLF and bLFC increased production of IFN-α by plasmacytoid dendritic cells (pDCs) that are crucial for the antiviral response. No such effect was observed upon pDC treatment by bLF or bLFC alone, suggesting that LF modulates the immune system by promoting pDC activation upon viral recognition [143]. However, when LF-treated moDCs were matured with LPS or other TLR ligands, a tolerogenic phenotype with decreased expression of activation markers, decreased release of proinflammatory cytokines, and decreased stimulatory capacity toward cocultured T cells was observed [144,145]. This again indicates that LF may represent a strategy to block excessive activation of the immune system upon inflammation triggered by TLR ligation.
LF also plays a role in balancing the potentially harmful excessive inflammatory response by acting as a negative feedback mechanism for neutrophils. It was observed that LF suppresses the release of neutrophil extracellular traps (NETs) both in vitro and in vivo [146]. NETs are a crucial defense mechanism against invasion of pathogens, but their uncontrolled release is associated with the development and progression of autoimmune diseases, inflammatory diseases, such as sepsis, and thrombosis. Exogenous hLF shrunk the chromatin fibers found in released NETs, without affecting the generation of ROS, but this failed after blockade of the positive charge in the N-terminus of LF with heparin, suggesting that charge–charge interactions between LF and NETs were required for this function [146].
These in vitro findings are supported by results of in vivo experiments in various disease animal models. They generally correlate with the predicted protective effects of LF. For example, in a mouse gastritis model induced by Helicobacter pylori, a Gram-negative pathogen linked to gastritis, ulceration, and stomach cancer, hLF treatment on top of the standard therapy dramatically contributed to H. pylori eradication, reduced gastric inflammation, and decreased the level of proinflammatory cytokines in stomach tissue [147]. Similarly, in a mouse peritonitis model using a lethal dose of the methicillin-resistant Gram-positive bacterium Staphylococcus aureus (MRSA), treatment with mouse LF resulted in modest increase in survival, albeit with reduced bacteremia and decreased serum levels of proinflammatory cytokines IL-17, IL-6, and IL-1β [97]. In a mouse model of tuberculosis, orally given bLF showed therapeutic effects against the aggressive Mycobacterium tuberculosis strain Erdman [148]. LF-treated mice showed reduced mycobacterial burden in the lung tissue, reduced lung inflammation with decreased foamy macrophages, and increased numbers of T cells that were producing IFN-γ and IL-17; these cells were thought to mediate protection against the infection. Furthermore, LF did not affect M. tuberculosis growth in vitro, but it enhanced the killing of mycobacteria by IFN-γ-stimulated macrophages in an NO-dependent manner [148]. In a follow-up study, orally given recombinant hLF or its fusion with the Fc domain of human IgG2 to extend its half-life in circulation were also protective against lung-induced pathology caused by the intravenous injection of the major cell wall component of M. tuberculosis, trehalose 6,6’-dimycolate (TDM) [149]. In this case, hLF and especially its Fc-fusion protein limited the lung inflammation and granuloma formation and contributed to resolution of the lung pathology over time. This was attributed to better control of TDM-induced proinflammatory cytokine production (TNF-α and IL-1β) in the lungs of mice treated by both forms of hLF [150]. LF also exhibited beneficial activities in experimental allergic rhinitis and allergic asthma, induced by sensitization with pollen or ovalbumin [151,152,153]. Protective effects of intranasally or orally applied hLF were attributed to lowering allergen-induced ROS levels in bronchial epithelial cells by apo-LF, with subsequent inhibition of eosinophil accumulation and reduced inflammation in the lungs [151], or to skewing of the immune response from the Th2 and Th17 to the protective Th1 type, as evident from increased airway IFN-γ and reduced Th2 (IL-4, IL-5, IL-13) and Th17 (IL-17) cytokines, as well as reduced ovalbumin-specific antibodies [152,153]. Parallel in vitro experiments revealed that LF influenced the phenotype of DCs and their antigen-presenting capacity, which resulted in a dampened ovalbumin-specific T cell response [153]. Lastly, the protective role of LF in allergy might be supported by a correlative observation that allergic rhinitis patients exhibit lower serum LF levels compared to nonallergic individuals [154].
Apart from microbial- or allergen-induced inflammation, several studies have shown that administration of LF may reduce experimental inflammation in tissues affected by other inflammatory disorders, such as neurodegenerative diseases [112], rheumatoid arthritis [155], IBD [156], or hyperoxia-induced lung and kidney inflammation [157]. In agreement with all these notions, LF knockout mice are more susceptible to various inflammatory disorders associated with impaired pathways of immune responses in various disease models [158,159,160,161,162].
Similarly to full-length LF, LFC was also found to convey immunomodulatory effects, i.e., by reducing the expression of proinflammatory cytokines and/or increasing the expression of anti-inflammatory cytokines [128,163,164,165,166]. In addition, it has also been shown that oral administration of the probiotic bacterium Lactobacillus reuteri expressing recombinant LFchimera to newborn piglets increases their resistance to enterotoxigenic E. coli (ETEC) strain K88 and diarrhea. LFchimera improves the piglet anti-inflammatory ability by inhibiting the NF-κB pathway, DC maturation, and T-cell activation, while also increasing their antioxidant capacity by activating the nuclear factor (erythroid-derived 2)-like 2 (NRF2)/heme oxygenase (HO-1) pathway, the master pathway that protects against oxidative damage caused by injury and inflammation [119,167]. Recently, it was confirmed that bLFC has anti-inflammatory properties by observing that it reduces the production of proinflammatory cytokines TNF-α and IL-6 in human and mouse macrophages upon stimulation with LPS. In particular, bLFC targeted the LPS-activated NF-κB and mitogen-activated protein kinase (MAPK) signaling pathways [168].
Lastly, it was shown that rectal administration of bLF did activate the bovine mucosal immune system and induce protection against enterohemorrhagic Escherichia coli (EHEC), likely through specific mucosal IgA [169]. Interestingly, the translocation of bLF into the nucleus of bovine rectal epithelial cells was observed in vitro upon inoculation with EHEC [170]. In this respect, it has been suggested that nucleolin together with proteoglycans mediates internalization of LF [66] (see also Section 2.3). Nevertheless, further analyses are needed to validate whether the nuclear translocation plays a role in the protective effects of bLF.
In summary, these studies showed that LF, LFC, and other LF-derived peptides display potent immunomodulatory functions to prevent infection- or inflammation-induced pathology (Figure 2). They predominantly target cells of the innate immune system (especially DCs, monocytes, and macrophages), which further influence cells of the adaptive immune system (T cells and B cells). However, very few studies pinpointed the action of LF to signaling through a particular LF receptor; so far, mainly actions via TLR4 have been studied [62,75,131,142]. Therefore, more research is needed to unravel the receptors and linked intracellular signaling pathways via which immunomodulatory actions of LF are executed.
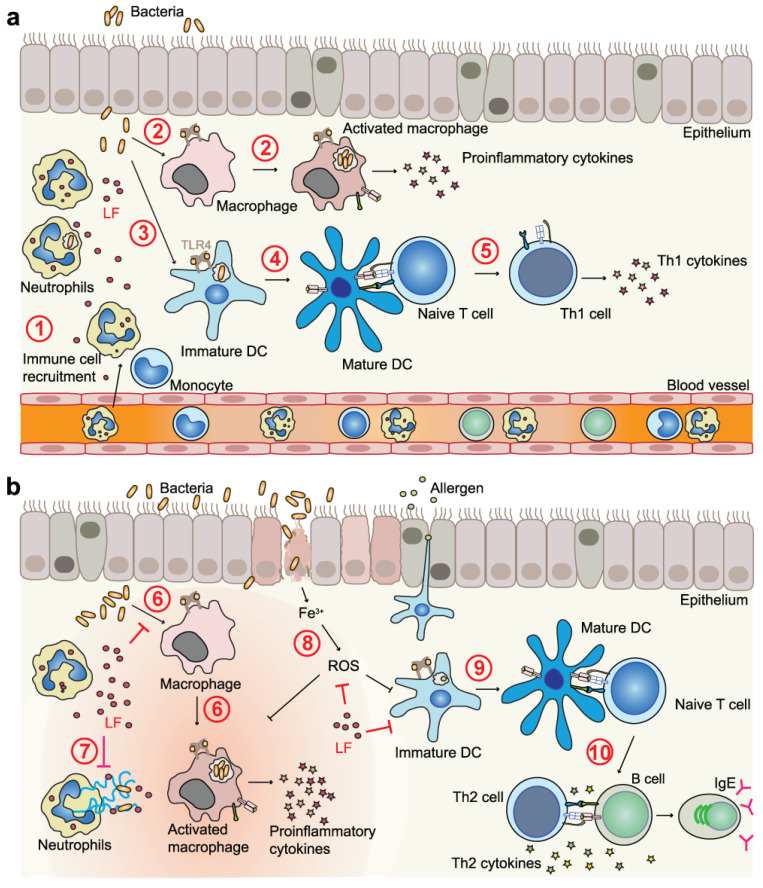
Figure 2.
Modulation of cells of the innate and adaptive immune system by LF contributes to host defense. (a) In early phases of the immune response to invading pathogens, LF released from neutrophil granules has an immunostimulatory role because of its function as an alarmin to mediate immune cell recruitment from the bloodstream to the affected tissue (1) and by activation of myeloid cells—macrophages (2) and DCs (3) in particular. These LF actions might be in part related to its ability to serve as a PAMP carrier. Lastly, by promoting DC maturation (4), LF has a positive influence on functions of the adaptive immune system by activating, for instance, T cells (5). (b) In contrast, in chronic infection or inflammation (e.g., in allergic inflammation), LF exerts immunomodulatory functions and, therefore, protective effects via several mechanisms. LF efficiently counteracts the excessive proinflammatory responses of macrophages triggered by PAMPs (6) and shrinks neutrophil extracellular traps (7). LF also efficiently scavenges free ferric iron, thereby blocking deleterious ROS production (8). In allergy, LF is known to modulate DC functions (9), thereby skewing the immune response from the harmful Th2 to the protective Th1 type (10) that is accompanied by reduced production of allergen-specific IgE antibodies by B cells.
4.3. LF and LFC as Inhibitors of Serine Proteases
Proteases exert essential functions in many physiological processes. Components of a plethora of proteolytic systems coordinate their activities to maintain homeostasis; yet, due to their enzymatic activities they can also contribute to a variety of human diseases. For example, the serine protease plasminogen is best characterized for its functions in fibrinolysis (resolution of blood clots) and cell migration. Plasminogen is also essential for immune cells to target pathogens or for endothelial cells to form vessels [171]. Nevertheless, human plasminogen might be also harnessed by malignant cancer cells and various pathogens. For instance, Borrelia burgdorferi hijacks plasminogen in a urokinase-type plasminogen activator (uPA)-dependent manner to increase invasiveness [172,173]. Streptococcus pyogenes secretes streptokinase capable of activating plasminogen independently of the host uPA [174]. Moreover, plasminogen and other serine proteases are implicated in proteolytic priming of various viruses [175]; the serine protease transmembrane protease serine type 2 (TMPRSS2) plays a decisive role in the proteolytic cleavage of the spike protein facilitating SARS-CoV-2 entry into host cells [176], while plasmin mediates the proteolytic processing of the H1N1 influenza virus hemagglutinin [177]. Hence, inhibitors of virus-priming serine proteases are a promising approach to manage virus infections.
In this regard, evidence has been gathered by us that LF directly binds plasminogen via its cationic N-terminus and reduces its proteolytic activity [71]. Furthermore, LF blocks the activation of plasminogen by Borrelia [71]. Moreover, LFC endows inhibitory potential toward plasminogen. Notably, LFC but not full-length LF is capable of blocking not only plasminogen conversion to plasmin but also the intrinsic plasmin proteolytic activity per se [178]. This difference between LF and LFC very likely stems from the above-described structural difference of free LFC from the N-terminal part within the whole molecule (see Figure 1). In addition, LFC blocks also TMPRSS2, which is homologous to plasminogen [178].
4.4. Direct Antiviral Activities of LF and LFC
Both hLF and bLF (and the respective LFCs, if tested) can block cell entry of many enveloped and naked viruses (Table 2) [11,179]. Through its N-terminal region, LF can bind to virus receptors (or coreceptors) on target mammalian cells, as well as block the initial stages of infection by several viruses, such as human immunodeficiency virus 1 (HIV-1) [58,64,73,76,180], dengue virus [181], herpes simplex virus 1 and 2 (HSV-1, HSV-2) [182,183], human and rat cytomegaloviruses (HCMV and RCMV), and several human coronaviruses (HCoVs, namely SARS-CoV-2, SARS-CoV-1, HCoV-229E, HCoV-NL63, and HCoV-OC43) [184,185,186]. In this respect, HSPGs as well-known and broadly expressed LF binding partners are of particular importance (Table 2) [30,54].
Table 2.
Role of LF and LFC in inhibition of virus infection by targeting the virus or viral (co)receptors.
| Virus | Mechanism of Action | Selected References |
|---|---|---|
| HIV-1 | Blockade of viral coreceptor CXCR4 of CXCR4-tropic HIV-1 strain by hLF | [58,73] |
| Inhibition of HIV-1 transfer from DCs to CD4 T cells by blockade of DC-SIGN (CD209) by bLF or hLF (and partially also by hLFC) | [64,76,180] | |
| HCMV, RCMV | Blockade of viral cell entry by hLF, bLF, hLFC, or bLFC, likely via interaction with HSPGs on target cells | [92,187] |
| Dengue virus | Blockade of viral coreceptors HSPGs, LRP-1 (CD91) and DC-SIGN (CD209) by bLF | [181] |
| HSV-1, HSV-2 | Blockade of HSPGs by hLF, bLF, hLFC, or bLFC; LFCs are more potent than LFs because of other mechanisms employed | [90,182,183] |
| Zika and Chikungunya viruses | Blockade of viral cell entry by bLF, probably via interaction with HSPGs on target cells | [188] |
| SARS-CoV-2 and other HCoVs | Inhibition of SARS-CoV-2, SARS-CoV-1, and other HCoV attachment because of the HSPG blockade by hLF or bLF | [184,185,186] |
| Blockade of SARS-CoV-2 S protein by hLF or bLF | [189] | |
| HCV | Direct blockade of HCV virions by bLF and hLF | [190] |
It has been observed that both bLF and bLFC are effective against HSV-1 and HSV-2 infection in vitro, with bLFC being more potent [182,183]. Furthermore, only bLFC administered therapeutically in a mouse model of HSV-2 genital infection was able to delay the onset of infection and significantly reduce the viral load, while bLF was not effective [183]. This suggests that the bioactive peptide might exert an antiviral activity via different mechanisms than the intact protein, due to differences in the 3D structure (see Figure 1). In particular, it was demonstrated that, although both LF and LFC can block viral entry via interaction with HSPGs, only LFC but not LF maintains its antiviral activity after the virus enters the cell [182]. This might be attributed to the specific properties of free LFC, such as the ability to enter the cell, as well as the higher capacity to induce an immune response in vivo [182,183].
To prevent human hepatitis C virus (HCV) infection, a direct binding of bLF or hLF to viral envelope proteins, rather than a blockade of a viral cell surface receptor is necessary [190]. Further research has shown that the interaction between the viral E2 protein and the C-terminal part of hLF, bLF, and horse LF was responsible for this effect [191].
Recently, LF has gained significant attention as studies have shown that LF and LF-derived peptides may inhibit the infection of SARS-CoV-2, the virus responsible for COVID-19, through the following mechanisms (Figure 3) [18,192]:
Through blocking the interaction between the viral S protein and HSPGs on the membrane of target cells by binding to HSPGs [184,185]. Interestingly, LF was observed to bind to HSPGs via its N-terminal region and inhibit cell infection of several HCoVs [185];
Through blockade of the TMPRSS2-mediated virus priming [178]. This mechanism has been observed in particular for LFC, both synthetic and natural, but not for full-length LF, again pointing to differences between the released LFC and the corresponding region encompassed within the N-terminus of intact LF;
Through blockade of the cathepsin L (CTSL)-mediated virus priming [194]. In this case, a bLF hydrolysate showed an inhibitory effect toward CTSL (a cysteine protease that primes SARS-CoV-2 in endosomes) that resulted in a decreased infection rate by a SARS-CoV-2 pseudovirus;
Through enhancement of IFN responses [195]. It has been shown that bLF enhances the expression of IFN-β and downstream IFN-stimulated genes (e.g., MX1 and IFITM3), all of which are known to exert antiviral effects;
Through inhibition of viral replication [196], which is due to direct inhibition of the viral RNA-dependent RNA polymerase (RdRp) activity by LF [197];
Through maintenance of iron homeostasis [198];
Possibly also through inhibition of the main viral protease Mpro, also called 3CLpro [199].
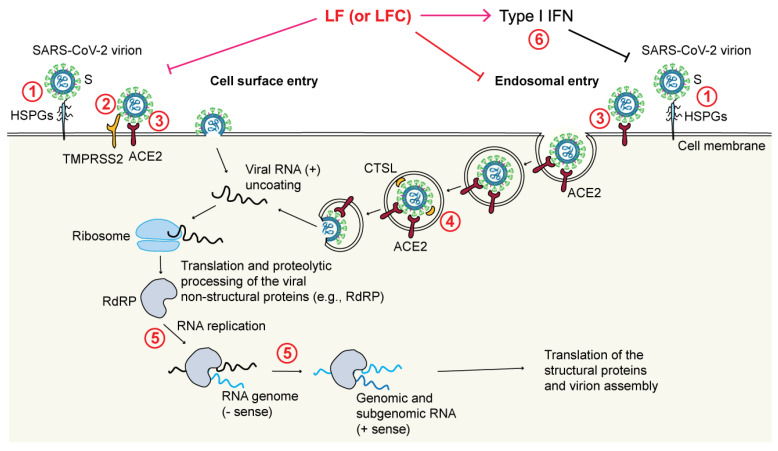
Figure 3.
LF and LFC can interfere with SARS-CoV-2 infection through various mechanisms. SARS-CoV-2 enters cells via two different routes: (i) via the cell surface, mediated by binding to HSPGs and ACE2 with S protein processing by the cell surface serine protease TMPRSS2, which is required for membrane fusion and subsequent infection, or (ii) via endosomes, in which HSPGs, ACE2, and the endosomal cysteine protease CTSL play a role. LF has been shown to interfere with both pathways, due to binding to HSPGs (1) or directly to the S protein (3), and due to inhibition of CTSL that cleaves the S protein to release the virus from the endosome (4). Furthermore, LF has been found to inhibit viral replication by directly targeting the viral RNA-dependent RNA polymerase (RdRP, 5). Lastly, LF can boost the antiviral response through enhancing type I IFNs (6). So far, LFC has been shown to inhibit viral entry by inhibition of the protease TMPRSS2 (2).
Furthermore, the antiviral activity of bLF and bLFC against several variants of SARS-CoV-2 has been studied in vitro [200]. In this case, bLF was more potent than bLFC, and the most profound effect of bLF was observed against the Alpha (B.1.1.7) variant. Different inhibition efficiencies of bLF onto individual SARS-CoV-2 variants are thought to be a result of their different affinities for HSPGs present on the surface of infected cells [200]. Taken together, these studies suggest that LF may be a potent, cost-effective, and widely available supplement in the management of COVID-19 due to its ability to target multiple stages of the virus life cycle.
4.5. Antibacterial, Antifungal, and Antiparasitic Activities of LF and LFC
LF has been shown to have a wide range of antibacterial effects (Figure 4), primarily due to its ability to sequester iron, as many pathogenic bacteria require iron for growth. These bacteriostatic properties of apo-LF are reversible, as bacterial growth can be restored by iron supplementation [11]. Nevertheless, the antimicrobial activities of LF are not only connected to iron deprivation [201]. LF has bactericidal effects due to binding to LPS, porins, and other outer membrane proteins of Gram-negative bacteria [121,202]. For example, LF binding to LPS causes LPS displacement and release, leading to the destabilization of the bacterial surface, depolarization of the outer bacterial membrane, and an increase in membrane permeability that ultimately results in enhanced bacterial susceptibility to osmotic shock, lysozyme, or antibiotics [203,204]. Although LF exerts its antimicrobial activities via two major skills, i.e., iron sequestration and selective binding, additional mechanisms via which LF impedes pathogens have been described.
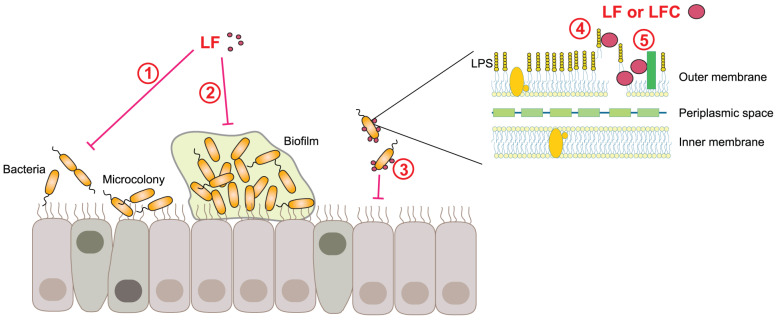
Figure 4.
Antimicrobial actions of LF and LFC. LF has antibacterial activity toward a spectrum of different bacterial pathogens, through iron sequestration, which is bacteriostatic (1), or leads to bacterial twitching that prevents biofilm formation (2). Moreover, via its N-glycans, LF acts as a soluble decoy receptor for invasive pathogens to disrupt their adherence to host cells and cell invasion strategies (3). LF and LFC can cause membrane permeabilization (4) and bind and neutralize bacterial virulence mechanisms, e.g., the type III secretion system of EPEC (5).
It has been observed that lowering the iron concentration in the environment by LF inhibits bacterial biofilm formation by Pseudomonas aeruginosa by promoting a specific movement called twitching. This movement prevents the bacteria from attaching to mammalian cell surfaces, forming microcolonies and eventually a biofilm. A fivefold lower concentration of LF is needed to promote twitching than required for its bacteriostatic activity [205]. LF also inhibits biofilm formation of Porphyromonas gingivalis via its antiproteinase activity [206]. Furthermore, LF has been shown to impair the type III secretion system important for virulence of the gastrointestinal bacterial pathogen enteropathogenic E. coli (EPEC), a common cause of infant diarrhea in developing countries [207]. Recently, it was also reported that bLF decreased motility of the flagellated ETEC strain of E. coli, which led to reduced bacterial colonization of the small intestinal epithelium [208]. Due to these multiple activities, LF is considered as the primary defense protein against microbial infections [12].
Concerning bLFCs and derived synthetic peptides, they are more potent than bLF, and act swiftly and directly on the plasma membrane of bacteria, causing leakage as a result of membrane permeabilization [209,210,211]. The presence of hydrophobic tryptophan residues and charged arginine residues in bLFC were proven to be of particular importance for its antibacterial activity [212]. A very short peptide derived from bLFC (RRWQWR) belongs to the category of so-called cell-penetrating antimicrobial peptides. These peptides enter the bacterial cell without disrupting the membrane to execute their antimicrobial function by a still unknown mechanism [213]. Although hLFC and derived peptides are considered less potent than bLFC (and its derivatives) in terms of pore formation and/or depolarization of the bacterial membrane, they can still serve as a supplementary treatment to enhance the effectiveness of antibiotics against anaerobic biofilm microbes [214].
In addition, bLFC and its synthetic derivatives were recently extensively explored as antifungal [215] and antiparasitic agents [216]. Similarly, synthetic peptides derived from the N-terminus of hLF showed a spectrum of activities against bacteria and fungi in vitro, as well as in animal models with peroral administration of the peptides [96,217,218,219,220]. These peptides acted synergistically with other antibacterials and antifungals and contributed to a reduction in the minimum concentration for inhibition [217,218,221]. Of note, synthetic hLFC (1–11) was also effective against multidrug-resistant strains of Staphylococcus aureus in an animal model of chronic osteomyelitis, offering a possibility for an antibiotic-independent treatment of these difficult-to-treat infections [222]. Thus, LFCs and their derived synthetic peptides not only conserve some of the properties of LF but may also provide a synergetic potential and convey augmented antimicrobial effects, intrinsic to their altered conformations (Figure 1).
However, pathogens have developed host-specific strategies to neutralize the effects of LF and LFC. For example, Streptococcus pneumoniae, a Gram-positive bacterium and an obligate human pathogen, can bind hLF via its major virulence factor pneumococcal surface protein A (PspA). PspA has a stronger binding affinity for hLF than bLF [223]. PspA does not need to be attached to the bacterial surface to neutralize hLF, as soluble recombinant PspA can also bind hLF and protect the bacteria from the bactericidal activity of apo-hLF [223]. Using recombinant peptides, the hLF bactericidal capacity was mapped to two stretches of hLFC—peptides hLFC (1–11) and hLFC (21–31), but only the first peptide hLFC (1–11) was strongly neutralized by the soluble PspA, thus mapping the interaction of PspA to the very N-terminal part of hLF [223]. Another way in which pathogens can evade the effects of LF is through the synthesis of specific proteins that bind to holo-LF in order to extract iron and subvert the nutritional immunity. For instance, certain Gram-negative bacteria in the Neisseriaceae family utilize a two-component system, consisting of the outer membrane lipoprotein termed lactoferrin-binding protein B (LbpB) and the membrane transporter termed lactoferrin-binding protein A (LbpA). To extract iron from holo-hLF, LbpB interacts with hLF in a 1:1 ratio and delivers it to LbpA [54,224,225,226]. Furthermore, LbpB interaction with hLFC-derived synthetic peptide hLFC (1–11) protects the bacterium against its killing activity [227]. A recent structural study revealed that holo-hLF and hLFC (1–11) interact with LbpB in a noncompetitive manner; the C-lobe of hLF binds to the LbpB N-lobe, whereas hLFC (1–11) binds negatively charged patches in the LbpB C-lobe. Thus, LbpB allows the bacteria to execute two actions: to obtain iron for growth while neutralizing the antimicrobial hLFC peptide [226]. Recently, these bacterial proteins that neutralize LF have been targeted as a potential solution for vaccines against colonization and invasive infections. This strategy may also help to prevent the selection and emergence of strains that can steal iron from the host LF [228].
4.6. Antitumor Activities of LF and LFC
In addition to its antimicrobial and immunomodulatory activities, a plethora of antitumor properties have been ascribed to LF, including the inhibition of tumor cell growth and metastasis, as well as a protective effect against carcinogenesis [229,230].
LF may exert its antitumor activities directly through the inhibition of tumor cell proliferation, e.g., by induction of cell-cycle arrest at the G1/S transition through modulation of the expression of cell-cycle-regulatory proteins [231,232]. Recombinant hLF has also been shown to induce apoptosis-related morphological changes, disruption of the cytoskeletal structure, phosphatidylserine externalization, cell-cycle arrest, and selective cytotoxicity in triple-negative breast cancer cells [233]. In addition, LF has been found to inhibit signaling pathways that promote tumor growth, such as the MAPK and AKT pathways [229,234], and it can induce cancer cell apoptosis by increasing the expression of Fas death receptors and activating the Fas death-inducing signaling complex and the caspase cascade [235]. Furthermore, increased ROS production by bLF, leading to oxidative stress and subsequent apoptosis of human prostate and cervical cancer cells has been reported [236,237]. Several studies have also shown that LF inhibits tumor progression through suppression of tumor angiogenesis [238,239,240]. Various mechanisms have been described, one of them involving the inhibition of the TNF receptor-associated factor 6 (TRAF6)/NF-κB pathway by bLF in tumor endothelial cells but not in nontumor endothelial cells. This led to suppression of hypoxia-inducible factor 1 α (HIF-1α) activation, reduced production of vascular endothelial growth factor A (VEGF-A), and decreased growth of tumor blood vessels [239]. Another important antitumor and antimetastatic effect of LF has been recognized in its inhibitory capacity toward cancer cell migration [241,242,243]. For the observed effect, e.g., a reversal of the epithelial-to-mesenchymal transition (EMT) in LF-treated cancer cells by downregulating the EMT marker SNAIL and upregulating cadherins has been identified as an underlying mechanism [241,243].
Additionally, LF may affect tumor growth and spread indirectly via its immunomodulatory mechanisms, by stimulating lymphocyte and natural killer (NK) cell activity. For example, oral administration of hLF increased the numbers of tumor-infiltrating CD4+ and CD8+ T cells in a mouse model of head and neck squamous cell carcinoma [244]. In a breast cancer mouse model, orally administered hLF enhanced intestinal IFN-γ production, which was associated with expansion of NKT cells and CD8+ T cells, as well as increased systemic cytotoxicity of tumor-specific CD8+ T cells [245]. Similarly, in a mouse model of cervical carcinoma expressing hLF, impaired tumor growth was associated with an increase in CD4+ and CD8+ T lymphocytes in peripheral blood [246]. Furthermore, studies in melanoma-bearing mice showed that LF deficiency led to increased lung metastasis through recruitment of myeloid-derived suppressor cells to the lungs and suppression of TLR9 signaling, which were reversible by oral treatment with LF, indicating an antimetastatic effect of LF [230].
Both hLF and bLF, when administered orally to mice with squamous cell carcinoma or basal-like breast cancer tumors, were found to enhance the effectiveness of conventional chemotherapy drugs, such as cisplatin and tamoxifen, and more effectively control the tumor growth [247,248]. The improved antitumor effects of this combination therapy were attributed to the systemic immune-stimulatory effect of IL-18 and IFN-γ, which were upregulated by the LF-stimulated gut enterocytes [247,248].
Recently, by virtue of its versatility in cellular binding, LF has been recognized and used as a targeting ligand for disease-bearing cells. In particular, LF-conjugated nanoparticles and direct LF–drug conjugates have been used to carry chemotherapeutics into cancer cells to overcome cancer chemoresistance and halt tumor development in experimental animal models [249,250].
It is worth noting that LF has been shown to be downregulated in many tumor cell lines, animal models, and human cancer tissues, including breast, nasopharyngeal, and colorectal cancer [160,229,230,234]. LF deficiency has been associated with the development of inflammation-triggered carcinogenesis, as well as with enhanced tumor progression and metastasis [160,230,251], suggesting that LF may play a role as a tumor suppressor.
bLFC, hLFC and peptides derived from them have been shown to induce apoptosis in cancer cells by targeting outer leaflet-exposed plasma membrane phosphatidylserine [252], by inducing intracellular ROS and activating of Ca2+/Mg2+-dependent endonucleases [253], by activating the p53 signaling pathway and the caspase cascade [254], or by inhibiting autophagy at the late stage [255]. Additionally, bLFC has been reported to reverse cisplatin resistance in head and neck squamous cell carcinoma through downmodulation of PD-L1 expression [256].
5. LF in Clinical Trials
Over decades, LF and LFC have become well known for their multiple activities in host defense responses [11,12,13,18,46]. In addition to many in vitro and in vivo studies outlined above, LF has been examined in several clinical trials that focused on its potential application in the treatment of a variety of human diseases. This is possible because the European Food Safety Authority (EFSA, 2012) [257] and the US Food and Drug Administration (FDA, 2014; GRAS notice, GRN 465) recognized the use of bLF as a food supplement and as a part of infant formula as generally safe.
One of the most promising areas of LF usage in practice is neonatal medicine. Neonatal sepsis can have serious, even lethal consequences, and it poses a problem especially in developing countries. Preterm and/or very-low-birth-weight (<1500 g) infants are in particular danger [258]. Several studies have demonstrated that orally administered bLF plays a critical role in protecting newborns, especially preterm and low-birth-weight ones, from infections, bacterial and fungal sepsis, or necrotizing enterocolitis [259,260,261,262]. However, a similar clinical trial in very preterm infants performed in the United Kingdom did not find significant differences in the incidence of late-onset infection between bLF and placebo groups [263]. Another clinical trial in term infants who were formula-fed, conducted in Sweden, also found no significant differences in various immunological parameters or infection rates between infants whose formula was supplemented with bLF or not [264]. It was concluded that bLF supplementation was safe but with no additional benefit in this privileged population with low infection burden, and further studies in more vulnerable populations are warranted [264].
As a prevention or treatment against diarrhea, bLF or hLF has also been tested in several randomized controlled clinical trials with older probands. A clinical trial from Peru involving weaned toddlers, enrolled at 12–18 months and followed for 6 months, showed that oral bLF supplementation decreased longitudinal prevalence and severity of diarrhea over placebo, despite no decrease in diarrhea incidence [265]. A second clinical trial in Peru examined the effects of adding recombinant hLF and lysozyme to a rice-based oral rehydration solution for children aged 5–33 months with acute diarrhea. The results showed that the addition of hLF and lysozyme significantly reduced diarrhea duration and severity, as well as prevented new diarrhea episodes [266]. Additionally, a small clinical trial performed in long-term care elderly patients demonstrated that recombinant hLF was effective over placebo in prevention of antibiotic-associated diarrhea during an 8-week observation period [267].
In a clinical trial for Helicobacter pylori eradication, oral supplementation of bLF twice daily for 15 days on top of the standard treatment resulted in more efficient eradication of the pathogen in treated patients [268]. Some double-blinded randomized placebo-controlled clinical trials also focused on possible application of bLF in dental hygiene and the prevention of periodontitis. For example, a long-term daily intake of tablets containing high-dose bLF (60 mg/day) in combination with lactoperoxidase and glucose oxidase for 12 weeks was associated with an improvement in oral health-related quality of life and prevention of periodontitis in healthy adults [269].
LF may serve as a prophylactic agent against respiratory tract infections. Several randomized clinical trials, recently summarized in the meta-analysis by Ali et al. [270], found that a dietary intake of bLF, as fortified formula, oral dietary supplement, or oral gel, was associated with decreased incidence of upper- or lower-respiratory tract infections in infants, children, or adult subjects.
In oncology, a blinded randomized controlled clinical trial evaluated the effects of oral bLF on the growth of colorectal polyps, likely to be adenomas, over a period of 1 year [271,272]. The results showed that the highest dose of bLF halted the growth of polyps. Furthermore, participants with regressing polyps showed increased NK cell activity, elevated serum hLF levels, indicative of increased neutrophil activity, and higher numbers of polyp-infiltrating CD4+ T cells. These findings suggest that bLF suppresses colorectal polyps by enhancing immune responses [272]. Recently, a double-blind placebo-controlled trial demonstrated the benefit of oral bLF supplementation during induction chemotherapy in pediatric patients with hematologic malignancies, associated with promotion of gut microbiome homeostasis [273]. LF supplementation also seems to mitigate taste and smell abnormalities among cancer patients receiving chemotherapy, which are caused by chemotherapy-induced lipid peroxidation effects in the oral cavity [274].
bLF also seems to have a potential as a supplementary treatment of iron deficiency and iron deficiency anemia in both pregnant and nonpregnant women of childbearing age [275], as well as of iron deficiency anemia in children with IBD [276]. Treatment with bLF (with iron saturation, if stated, of about 30%) was well tolerated and more effective than treatment with ferrous sulfate in restoring iron homeostasis in both anemic women and children. This resulted in improvement of all hematological parameters measured (hematocrit, hemoglobin, total serum iron, and serum ferritin) and normalization of serum levels of IL-6 and of hepcidin, the central regulator of systemic iron homeostasis [275,276].
Last, but not least, LF has gained profound attention as a potential supplemental treatment for COVID-19. In this respect, two small studies were performed in Italy. In the first nonrandomized retrospective study, asymptomatic to moderately symptomatic COVID-19 patients who took bLF as a nutraceutical, or not, were followed up. Faster viral clearance and more pronounced reduction in symptoms with increasing patient age were observed in the group with oral bLF supplementation [277]. In the second trial, asymptomatic to mild-to-moderate COVID-19 patients were assigned to three groups: a mix of hospitalized and home-treated patients received oral and intranasal liposomal bLF, the second hospitalized group received standard-of-care treatment, and the third group of home-treated patients did not take any medication. It was reported that liposomal bLF treatment was without adverse effects and led to faster recovery with decreased serum ferritin, IL-6, and D-dimers, compared to standard-of-care-treated patients [278]. These studies suggest that LF might be beneficial as a supplementary therapy against COVID-19. In contrast, a recently published double-blinded randomized control trial found no link between bLF supplementation and prevention of COVID-19 in healthcare workers. In a few participants who got infected and took bLF versus placebo, no significant differences in severity and duration of symptomatic infection were observed [279]. Similarly, a small randomized prospective interventional pilot study from Egypt showed no statistically significant differences regarding recovery of symptoms or improvement of several laboratory parameters measured in mild-to-moderate COVID-19 patients who took supplemental LF or not [280]. In conclusion, larger randomized clinical trials focused on differential timing, dosage, and duration of the treatment with LF are needed to validate the potential beneficial roles for supplemental treatment of COVID-19.
6. Conclusions
LF is a multifaceted protein that plays a critical role in host defense. Its unique structural properties, such as the ability to sequester iron and the selective binding via its highly basic N-terminus, allow it to function as both a component of innate immune responses and a modulator of adaptive responses. By virtue of these capabilities, LF performs a wide range of immune functions, ranging from direct killing of pathogens to modulation of inflammation. Similarly, LFCs of various origin can bind to various targets, such as negatively charged lipids in the membranes of bacteria and lower eukaryotes or the outer leaflet of cancer cells. Thus, both LF and LFC are highly valuable natural substances with a range of potential health benefits that can balance between pathogen elimination and promoting healing, in line with the statement in Ecclesiastes 3:3, when it is “a time to kill and a time to heal”.
Acknowledgments
Open Access Funding by the Austrian Science Fund (FWF).
Author Contributions
Conceptualization, A.O.-R., H.S. and V.L.; writing—original draft preparation, A.O.-R., R.P., T.M., L.G., R.S., O.C., G.T., H.S. and V.L.; writing—review and editing, A.O.-R., R.P., L.G., G.T., R.S., O.C., V.L. and H.S.; visualization, A.O.-R., R.S., H.S. and O.C.; supervision, A.O.-R. and V.L.; project administration, A.O.-R. and V.L.; funding acquisition, A.O.-R., H.S. and V.L. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
The data presented in this study are available in this article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by grants of the Austrian Science Fund (FWF; P 34253-B), the Science and Technology Assistance Agency of the Slovak Republic (APVV-20-0513), and the Slovak Grant Agency VEGA (2/0152/21).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Liao Y., Alvarado R., Phinney B., Lonnerdal B. Proteomic characterization of human milk whey proteins during a twelve-month lactation period. J. Proteome Res. 2011;10:1746–1754. doi: 10.1021/pr101028k. [DOI] [PubMed] [Google Scholar]
- 2.Ballard O., Morrow A.L. Human milk composition: Nutrients and bioactive factors. Pediatr. Clin. N. Am. 2013;60:49–74. doi: 10.1016/j.pcl.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chong H.Y., Tan L.T., Law J.W., Hong K.W., Ratnasingam V., Ab Mutalib N.S., Lee L.H., Letchumanan V. Exploring the Potential of Human Milk and Formula Milk on Infants’ Gut and Health. Nutrients. 2022;14:3554. doi: 10.3390/nu14173554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.El-Fakharany E.M. Nanoformulation of lactoferrin potentiates its activity and enhances novel biotechnological applications. Int. J. Biol. Macromol. 2020;165:970–984. doi: 10.1016/j.ijbiomac.2020.09.235. [DOI] [PubMed] [Google Scholar]
- 5.Baker E.N., Baker H.M. A structural framework for understanding the multifunctional character of lactoferrin. Biochimie. 2009;91:3–10. doi: 10.1016/j.biochi.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 6.Lambert L.A. Molecular evolution of the transferrin family and associated receptors. Biochim. Biophys. Acta. 2012;1820:244–255. doi: 10.1016/j.bbagen.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Sorensen M., Sorensen S.P.L. The proteins in whey. C. R. Des. Trav. Lab. Carlsberg Ser. Chim. 1940;23:55–99. [Google Scholar]
- 8.Manzoni P. Clinical Benefits of Lactoferrin for Infants and Children. J. Pediatr. 2016;173:S43–S52. doi: 10.1016/j.jpeds.2016.02.075. [DOI] [PubMed] [Google Scholar]
- 9.Giansanti F., Panella G., Leboffe L., Antonini G. Lactoferrin from Milk: Nutraceutical and Pharmacological Properties. Pharmaceuticals. 2016;9:61. doi: 10.3390/ph9040061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boukria O., El Hadrami E.M., Sameen A., Sahar A., Khan S., Safarov J., Sultanova S., Leriche F., Ait-Kaddour A. Biochemical, Physicochemical and Sensory Properties of Yoghurts Made from Mixing Milks of Different Mammalian Species. Foods. 2020;9:1722. doi: 10.3390/foods9111722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zarzosa-Moreno D., Avalos-Gomez C., Ramirez-Texcalco L.S., Torres-Lopez E., Ramirez-Mondragon R., Hernandez-Ramirez J.O., Serrano-Luna J., de la Garza M. Lactoferrin and Its Derived Peptides: An Alternative for Combating Virulence Mechanisms Developed by Pathogens. Molecules. 2020;25:5763. doi: 10.3390/molecules25245763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kruzel M.L., Zimecki M., Actor J.K. Lactoferrin in a Context of Inflammation-Induced Pathology. Front. Immunol. 2017;8:1438. doi: 10.3389/fimmu.2017.01438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Legrand D., Mazurier J. A critical review of the roles of host lactoferrin in immunity. Biometals. 2010;23:365–376. doi: 10.1007/s10534-010-9297-1. [DOI] [PubMed] [Google Scholar]
- 14.Vogel H.J. Lactoferrin, a bird’s eye view. Biochem. Cell Biol. 2012;90:233–244. doi: 10.1139/o2012-016. [DOI] [PubMed] [Google Scholar]
- 15.Kowalczyk P., Kaczynska K., Kleczkowska P., Bukowska-Osko I., Kramkowski K., Sulejczak D. The Lactoferrin Phenomenon-A Miracle Molecule. Molecules. 2022;27:2941. doi: 10.3390/molecules27092941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Embleton N.D., Berrington J.E. Clinical Trials of Lactoferrin in the Newborn: Effects on Infection and the Gut Microbiome. Nestle Nutr. Inst. Workshop Ser. 2020;94:141–151. doi: 10.1159/000505334. [DOI] [PubMed] [Google Scholar]
- 17.Artym J., Zimecki M. Milk-derived proteins and peptides in clinical trials. Postep. Hig. Med. Dosw. 2013;67:800–816. doi: 10.5604/17322693.1061635. [DOI] [PubMed] [Google Scholar]
- 18.Rosa L., Cutone A., Conte M.P., Campione E., Bianchi L., Valenti P. An overview on in vitro and in vivo antiviral activity of lactoferrin: Its efficacy against SARS-CoV-2 infection. Biometals. 2022:1–20. doi: 10.1007/s10534-022-00427-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gonzalez-Chavez S.A., Arevalo-Gallegos S., Rascon-Cruz Q. Lactoferrin: Structure, function and applications. Int. J. Antimicrob. Agents. 2009;33:e301–e308. doi: 10.1016/j.ijantimicag.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 20.Anderson B.F., Baker H.M., Dodson E.J., Norris G.E., Rumball S.V., Waters J.M., Baker E.N. Structure of human lactoferrin at 3.2-A resolution. Proc. Natl. Acad. Sci. USA. 1987;84:1769–1773. doi: 10.1073/pnas.84.7.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson B.F., Baker H.M., Norris G.E., Rumball S.V., Baker E.N. Apolactoferrin structure demonstrates ligand-induced conformational change in transferrins. Nature. 1990;344:784–787. doi: 10.1038/344784a0. [DOI] [PubMed] [Google Scholar]
- 22.Ianiro G., Rosa L., Bonaccorsi di Patti M.C., Valenti P., Musci G., Cutone A. Lactoferrin: From the structure to the functional orchestration of iron homeostasis. Biometals. 2022:1–26. doi: 10.1007/s10534-022-00453-x. [DOI] [PubMed] [Google Scholar]
- 23.Baker H.M., Baker E.N. Lactoferrin and iron: Structural and dynamic aspects of binding and release. Biometals. 2004;17:209–216. doi: 10.1023/B:BIOM.0000027694.40260.70. [DOI] [PubMed] [Google Scholar]
- 24.Baker E.N., Anderson B.F., Baker H.M., Haridas M., Norris G.E., Rumball S.V., Smith C.A. Metal and anion binding sites in lactoferrin and related proteins. Pure Appl. Chem. 1990;62:1067–1070. doi: 10.1351/pac199062061067. [DOI] [Google Scholar]
- 25.Anghel L., Radulescu A., Erhan R.V. Structural aspects of human lactoferrin in the iron-binding process studied by molecular dynamics and small-angle neutron scattering. Eur. Phys. J. E Soft Matter. 2018;41:109. doi: 10.1140/epje/i2018-11720-x. [DOI] [PubMed] [Google Scholar]
- 26.Majka G., Spiewak K., Kurpiewska K., Heczko P., Stochel G., Strus M., Brindell M. A high-throughput method for the quantification of iron saturation in lactoferrin preparations. Anal. Bioanal. Chem. 2013;405:5191–5200. doi: 10.1007/s00216-013-6943-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ying L., Furmanski P. Iron binding to human lactoferrin alters reactivity of the protein with plant lectins. Biochem. Biophys. Res. Commun. 1993;196:686–691. doi: 10.1006/bbrc.1993.2304. [DOI] [PubMed] [Google Scholar]
- 28.Aisen P., Leibman A. Lactoferrin and transferrin: A comparative study. Biochim. Biophys. Acta Protein Struct. 1972;257:314–323. doi: 10.1016/0005-2795(72)90283-8. [DOI] [PubMed] [Google Scholar]
- 29.Van Berkel P.H., Geerts M.E., van Veen H.A., Mericskay M., de Boer H.A., Nuijens J.H. N-terminal stretch Arg2, Arg3, Arg4 and Arg5 of human lactoferrin is essential for binding to heparin, bacterial lipopolysaccharide, human lysozyme and DNA. Biochem. J. 1997;328:145–151. doi: 10.1042/bj3280145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.El Yazidi-Belkoura I., Legrand D., Nuijens J., Slomianny M.C., van Berkel P., Spik G. The binding of lactoferrin to glycosaminoglycans on enterocyte-like HT29-18-C1 cells is mediated through basic residues located in the N-terminus. Biochim. Biophys. Acta Gen. Subj. 2001;1568:197–204. doi: 10.1016/S0304-4165(01)00222-7. [DOI] [PubMed] [Google Scholar]
- 31.Zlatina K., Galuska S.P. The N-glycans of lactoferrin: More than just a sweet decoration. Biochem. Cell Biol. 2021;99:117–127. doi: 10.1139/bcb-2020-0106. [DOI] [PubMed] [Google Scholar]
- 32.Spik G., Coddeville B., Montreuil J. Comparative study of the primary structures of sero-, lacto- and ovotransferrin glycans from different species. Biochimie. 1988;70:1459–1469. doi: 10.1016/0300-9084(88)90283-0. [DOI] [PubMed] [Google Scholar]
- 33.Barboza M., Pinzon J., Wickramasinghe S., Froehlich J.W., Moeller I., Smilowitz J.T., Ruhaak L.R., Huang J., Lonnerdal B., German J.B., et al. Glycosylation of human milk lactoferrin exhibits dynamic changes during early lactation enhancing its role in pathogenic bacteria-host interactions. Mol. Cell. Proteom. 2012;11:M111 015248. doi: 10.1074/mcp.M111.015248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Valk-Weeber R.L., Eshuis-de Ruiter T., Dijkhuizen L., van Leeuwen S.S. Dynamic Temporal Variations in Bovine Lactoferrin Glycan Structures. J. Agric. Food Chem. 2020;68:549–560. doi: 10.1021/acs.jafc.9b06762. [DOI] [PubMed] [Google Scholar]
- 35.Van Berkel P.H.C., Geerts M.E.J., Vanveen H.A., Kooiman P.M., Pieper F.R., Deboer H.A., Nuijens J.H. Glycosylated and Unglycosylated Human Lactoferrins Both Bind Iron and Show Identical Affinities towards Human Lysozyme and Bacterial Lipopolysaccharide, but Differ in Their Susceptibilities towards Tryptic Proteolysis. Biochem. J. 1995;312:107–114. doi: 10.1042/bj3120107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zheng F., Du Y.M., Lin X.S., Zhou L.Q., Bai Y., Yu X.B., Voglmeir J., Liu L. N-Glycosylation Plays an Essential and Species-Specific Role in Anti-Infection Function of Milk Proteins Using Listeria monocytogenes as Model Pathogen. J. Agric. Food Chem. 2019;67:10774–10781. doi: 10.1021/acs.jafc.9b03154. [DOI] [PubMed] [Google Scholar]
- 37.Ward P.P., Mendoza-Meneses M., Mulac-Jericevic B., Cunningham G.A., Saucedo-Cardenas O., Teng C.T., Conneely O.M. Restricted spatiotemporal expression of lactoferrin during murine embryonic development. Endocrinology. 1999;140:1852–1860. doi: 10.1210/endo.140.4.6671. [DOI] [PubMed] [Google Scholar]
- 38.Yanaihara A., Mitsukawa K., Iwasaki S., Otsuki K., Kawamura T., Okai T. High concentrations of lactoferrin in the follicular fluid correlate with embryo quality during in vitro fertilization cycles. Fertil. Steril. 2007;87:279–282. doi: 10.1016/j.fertnstert.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 39.Teng C.T. Lactoferrin gene expression and regulation: An overview. Biochem. Cell Biol. 2002;80:7–16. doi: 10.1139/o01-215. [DOI] [PubMed] [Google Scholar]
- 40.Teng C.T., Gladwell W., Beard C., Walmer D., Teng C.S., Brenner R. Lactoferrin gene expression is estrogen responsive in human and rhesus monkey endometrium. Mol. Hum. Reprod. 2002;8:58–67. doi: 10.1093/molehr/8.1.58. [DOI] [PubMed] [Google Scholar]
- 41.Dikovskaya M.A., Trunov A.N., Chernykh V.V., Korolenko T.A. Cystatin C and lactoferrin concentrations in biological fluids as possible prognostic factors in eye tumor development. Int. J. Circumpolar Health. 2013;72:21087. doi: 10.3402/ijch.v72i0.21087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park J.H., Park G.T., Cho I.H., Sim S.M., Yang J.M., Lee D.Y. An antimicrobial protein, lactoferrin exists in the sweat: Proteomic analysis of sweat. Exp. Dermatol. 2011;20:369–371. doi: 10.1111/j.1600-0625.2010.01218.x. [DOI] [PubMed] [Google Scholar]
- 43.Sanchez L., Aranda P., Perez M.D., Calvo M. Concentration of lactoferrin and transferrin throughout lactation in cow’s colostrum and milk. Biol. Chem. Hoppe Seyler. 1988;369:1005–1008. doi: 10.1515/bchm3.1988.369.2.1005. [DOI] [PubMed] [Google Scholar]
- 44.Masson P.L., Heremans J.F. Lactoferrin in milk from different species. Comp. Biochem. Physiol. B. 1971;39:119–129. doi: 10.1016/0305-0491(71)90258-6. [DOI] [PubMed] [Google Scholar]
- 45.Welty F.K., Smith K.L., Schanbacher F.L. Lactoferrin concentration during involution of the bovine mammary gland. J. Dairy Sci. 1976;59:224–231. doi: 10.3168/jds.S0022-0302(76)84188-4. [DOI] [PubMed] [Google Scholar]
- 46.Drago-Serrano M.E., Campos-Rodriguez R., Carrero J.C., de la Garza M. Lactoferrin and Peptide-derivatives: Antimicrobial Agents with Potential Use in Nonspecific Immunity Modulation. Curr. Pharm. Des. 2018;24:1067–1078. doi: 10.2174/1381612824666180327155929. [DOI] [PubMed] [Google Scholar]
- 47.Berliner N., Hsing A., Graubert T., Sigurdsson F., Zain M., Bruno E., Hoffman R. Granulocyte colony-stimulating factor induction of normal human bone marrow progenitors results in neutrophil-specific gene expression. Blood. 1995;85:799–803. doi: 10.1182/blood.V85.3.799.bloodjournal853799. [DOI] [PubMed] [Google Scholar]
- 48.Masson P.L., Heremans J.F., Schonne E. Lactoferrin, an iron-binding protein in neutrophilic leukocytes. J. Exp. Med. 1969;130:643–658. doi: 10.1084/jem.130.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bennett R.M., Kokocinski T. Lactoferrin Turnover in Man. Clin. Sci. 1979;57:453–460. doi: 10.1042/cs0570453. [DOI] [PubMed] [Google Scholar]
- 50.Kane S.V., Sandborn W.J., Rufo P.A., Zholudev A., Boone J., Lyerly D., Camilleri M., Hanauer S.B. Fecal lactoferrin is a sensitive and specific marker in identifying intestinal inflammation. Am. J. Gastroenterol. 2003;98:1309–1314. doi: 10.1111/j.1572-0241.2003.07458.x. [DOI] [PubMed] [Google Scholar]
- 51.Zhou X.L., Xu W., Tang X.X., Luo L.S., Tu J.F., Zhang C.J., Xu X., Wu Q.D., Pan W.S. Fecal lactoferrin in discriminating inflammatory bowel disease from irritable bowel syndrome: A diagnostic meta-analysis. BMC Gastroenterol. 2014;14:121. doi: 10.1186/1471-230X-14-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fillebeen C., Ruchoux M.M., Mitchell V., Vincent S., Benaissa M., Pierce A. Lactoferrin is synthesized by activated microglia in the human substantia nigra and its synthesis by the human microglial CHME cell line is upregulated by tumor necrosis factor alpha or 1-methyl-4-phenylpyridinium treatment. Mol. Brain Res. 2001;96:103–113. doi: 10.1016/S0169-328X(01)00216-9. [DOI] [PubMed] [Google Scholar]
- 53.Bournazou I., Pound J.D., Duffin R., Bournazos S., Melville L.A., Brown S.B., Rossi A.G., Gregory C.D. Apoptotic human cells inhibit migration of granulocytes via release of lactoferrin. J. Clin. Investig. 2009;119:20–32. doi: 10.1172/JCI36226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kell D.B., Heyden E.L., Pretorius E. The Biology of Lactoferrin, an Iron-Binding Protein That Can Help Defend Against Viruses and Bacteria. Front. Immunol. 2020;11:1221. doi: 10.3389/fimmu.2020.01221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Takayama Y., Takahashi H., Mizumachi K., Takezawa T. Low density lipoprotein receptor-related protein (LRP) is required for lactoferrin-enhanced collagen gel contractile activity of human fibroblasts. J. Biol. Chem. 2003;278:22112–22118. doi: 10.1074/jbc.M300894200. [DOI] [PubMed] [Google Scholar]
- 56.Willnow T.E., Goldstein J.L., Orth K., Brown M.S., Herz J. Low density lipoprotein receptor-related protein and gp330 bind similar ligands, including plasminogen activator-inhibitor complexes and lactoferrin, an inhibitor of chylomicron remnant clearance. J. Biol. Chem. 1992;267:26172–26180. doi: 10.1016/S0021-9258(18)35732-6. [DOI] [PubMed] [Google Scholar]
- 57.Grey A., Banovic T., Zhu Q., Watson M., Callon K., Palmano K., Ross J., Naot D., Reid I.R., Cornish J. The low-density lipoprotein receptor-related protein 1 is a mitogenic receptor for lactoferrin in osteoblastic cells. Mol. Endocrinol. 2004;18:2268–2278. doi: 10.1210/me.2003-0456. [DOI] [PubMed] [Google Scholar]
- 58.Takayama Y., Aoki R., Uchida R., Tajima A., Aoki-Yoshida A. Role of CXC chemokine receptor type 4 as a lactoferrin receptor. Biochem. Cell Biol. 2017;95:57–63. doi: 10.1139/bcb-2016-0039. [DOI] [PubMed] [Google Scholar]
- 59.Shin K., Wakabayashi H., Yamauchi K., Yaeshima T., Iwatsuki K. Recombinant human intelectin binds bovine lactoferrin and its peptides. Biol. Pharm. Bull. 2008;31:1605–1608. doi: 10.1248/bpb.31.1605. [DOI] [PubMed] [Google Scholar]
- 60.Ward P.P., Mendoza-Meneses M., Cunningham G.A., Conneely O.M. Iron status in mice carrying a targeted disruption of lactoferrin. Mol. Cell. Biol. 2003;23:178–185. doi: 10.1128/MCB.23.1.178-185.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Baveye S., Elass E., Fernig D.G., Blanquart C., Mazurier J., Legrand D. Human lactoferrin interacts with soluble CD14 and inhibits expression of endothelial adhesion molecules, E-selectin and ICAM-1, induced by the CD14-lipopolysaccharide complex. Infect. Immun. 2000;68:6519–6525. doi: 10.1128/IAI.68.12.6519-6525.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Curran C.S., Demick K.P., Mansfield J.M. Lactoferrin activates macrophages via TLR4-dependent and -independent signaling pathways. Cell. Immunol. 2006;242:23–30. doi: 10.1016/j.cellimm.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 63.Zheng Y., Qin Z., Ye Q., Chen P., Wang Z., Yan Q., Luo Z., Liu X., Zhou Y., Xiong W., et al. Lactoferrin suppresses the Epstein-Barr virus-induced inflammatory response by interfering with pattern recognition of TLR2 and TLR9. Lab. Investig. 2014;94:1188–1199. doi: 10.1038/labinvest.2014.105. [DOI] [PubMed] [Google Scholar]
- 64.Carthagena L., Becquart P., Hocini H., Kazatchkine M.D., Bouhlal H., Belec L. Modulation of HIV Binding to Epithelial Cells and HIV Transfer from Immature Dendritic Cells to CD4 T Lymphocytes by Human Lactoferrin and its Major Exposed LF-33 Peptide. Open Virol. J. 2011;5:27–34. doi: 10.2174/1874357901105010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mazurier J., Legrand D., Hu W.L., Montreuil J., Spik G. Expression of human lactotransferrin receptors in phytohemagglutinin-stimulated human peripheral blood lymphocytes. Isolation of the receptors by antiligand-affinity chromatography. Eur. J. Biochem. 1989;179:481–487. doi: 10.1111/j.1432-1033.1989.tb14578.x. [DOI] [PubMed] [Google Scholar]
- 66.Legrand D., Vigie K., Said E.A., Elass E., Masson M., Slomianny M.C., Carpentier M., Briand J.P., Mazurier J., Hovanessian A.G. Surface nucleolin participates in both the binding and endocytosis of lactoferrin in target cells. Eur. J. Biochem. 2004;271:303–317. doi: 10.1046/j.1432-1033.2003.03929.x. [DOI] [PubMed] [Google Scholar]
- 67.Damiens E., El Yazidi I., Mazurier J., Elass-Rochard E., Duthille I., Spik G., Boilly-Marer Y. Role of heparan sulphate proteoglycans in the regulation of human lactoferrin binding and activity in the MDA-MB-231 breast cancer cell line. Eur. J. Cell Biol. 1998;77:344–351. doi: 10.1016/S0171-9335(98)80093-9. [DOI] [PubMed] [Google Scholar]
- 68.Thorne R.G., Lakkaraju A., Rodriguez-Boulan E., Nicholson C. In vivo diffusion of lactoferrin in brain extracellular space is regulated by interactions with heparan sulfate. Proc. Natl. Acad. Sci. USA. 2008;105:8416–8421. doi: 10.1073/pnas.0711345105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sokolov A.V., Ageeva K.V., Pulina M.O., Zakharova E.T., Vasilyev V.B. Effect of lactoferrin on oxidative features of ceruloplasmin. Biometals. 2009;22:521–529. doi: 10.1007/s10534-009-9209-4. [DOI] [PubMed] [Google Scholar]
- 70.Yamniuk A.P., Burling H., Vogel H.J. Thermodynamic characterization of the interactions between the immunoregulatory proteins osteopontin and lactoferrin. Mol. Immunol. 2009;46:2395–2402. doi: 10.1016/j.molimm.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 71.Zwirzitz A., Reiter M., Skrabana R., Ohradanova-Repic A., Majdic O., Gutekova M., Cehlar O., Petrovcikova E., Kutejova E., Stanek G., et al. Lactoferrin is a natural inhibitor of plasminogen activation. J. Biol. Chem. 2018;293:8600–8613. doi: 10.1074/jbc.RA118.003145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cappelletti M., Presicce P., Calcaterra F., Mavilio D., Della Bella S. Bright expression of CD91 identifies highly activated human dendritic cells that can be expanded by defensins. Immunology. 2015;144:661–667. doi: 10.1111/imm.12418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang J.F., Liu Z.Y., Groopman J.E. The alpha-chemokine receptor CXCR4 is expressed on the megakaryocytic lineage from progenitor to platelets and modulates migration and adhesion. Blood. 1998;92:756–764. doi: 10.1182/blood.V92.3.756. [DOI] [PubMed] [Google Scholar]
- 74.Ohradanova-Repic A., Machacek C., Fischer M.B., Stockinger H. Differentiation of human monocytes and derived subsets of macrophages and dendritic cells by the HLDA10 monoclonal antibody panel. Clin. Transl. Immunol. 2016;5:e55. doi: 10.1038/cti.2015.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ando K., Hasegawa K., Shindo K., Furusawa T., Fujino T., Kikugawa K., Nakano H., Takeuchi O., Akira S., Akiyama T., et al. Human lactoferrin activates NF-kappaB through the Toll-like receptor 4 pathway while it interferes with the lipopolysaccharide-stimulated TLR4 signaling. FEBS J. 2010;277:2051–2066. doi: 10.1111/j.1742-4658.2010.07620.x. [DOI] [PubMed] [Google Scholar]
- 76.Stax M.J., Mouser E.E., van Montfort T., Sanders R.W., de Vries H.J., Dekker H.L., Herrera C., Speijer D., Pollakis G., Paxton W.A. Colorectal mucus binds DC-SIGN and inhibits HIV-1 trans-infection of CD4+ T-lymphocytes. PLoS ONE. 2015;10:e0122020. doi: 10.1371/journal.pone.0122020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ohradanova-Repic A., Machacek C., Charvet C., Lager F., Le Roux D., Platzer R., Leksa V., Mitulovic G., Burkard T.R., Zlabinger G.J., et al. Extracellular Purine Metabolism Is the Switchboard of Immunosuppressive Macrophages and a Novel Target to Treat Diseases with Macrophage Imbalances. Front. Immunol. 2018;9:852. doi: 10.3389/fimmu.2018.00852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Troost F.J., Saris W.H., Brummer R.J. Orally ingested human lactoferrin is digested and secreted in the upper gastrointestinal tract in vivo in women with ileostomies. J. Nutr. 2002;132:2597–2600. doi: 10.1093/jn/132.9.2597. [DOI] [PubMed] [Google Scholar]
- 79.Kuwata H., Yip T.T., Tomita M., Hutchens T.W. Direct evidence of the generation in human stomach of an antimicrobial peptide domain (lactoferricin) from ingested lactoferrin. Biochim. Biophys. Acta. 1998;1429:129–141. doi: 10.1016/S0167-4838(98)00224-6. [DOI] [PubMed] [Google Scholar]
- 80.Brines R.D., Brock J.H. The effect of trypsin and chymotrypsin on the in vitro antimicrobial and iron-binding properties of lactoferrin in human milk and bovine colostrum. Unusual resistance of human apolactoferrin to proteolytic digestion. Biochim. Biophys. Acta. 1983;759:229–235. doi: 10.1016/0304-4165(83)90317-3. [DOI] [PubMed] [Google Scholar]
- 81.Troost F.J., Steijns J., Saris W.H., Brummer R.J. Gastric digestion of bovine lactoferrin in vivo in adults. J. Nutr. 2001;131:2101–2104. doi: 10.1093/jn/131.8.2101. [DOI] [PubMed] [Google Scholar]
- 82.Spik G., Brunet B., Mazurier-Dehaine C., Fontaine G., Montreuil J. Characterization and properties of the human and bovine lactotransferrins extracted from the faeces of newborn infants. Acta Paediatr. Scand. 1982;71:979–985. doi: 10.1111/j.1651-2227.1982.tb09560.x. [DOI] [PubMed] [Google Scholar]
- 83.Bellamy W., Takase M., Yamauchi K., Wakabayashi H., Kawase K., Tomita M. Identification of the bactericidal domain of lactoferrin. Biochim. Biophys. Acta. 1992;1121:130–136. doi: 10.1016/0167-4838(92)90346-F. [DOI] [PubMed] [Google Scholar]
- 84.Gifford J.L., Hunter H.N., Vogel H.J. Lactoferricin: A lactoferrin-derived peptide with antimicrobial, antiviral, antitumor and immunological properties. Cell. Mol. Life Sci. 2005;62:2588–2598. doi: 10.1007/s00018-005-5373-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bellamy W., Wakabayashi H., Takase M., Kawase K., Shimamura S., Tomita M. Killing of Candida albicans by lactoferricin B, a potent antimicrobial peptide derived from the N-terminal region of bovine lactoferrin. Med. Microbiol. Immunol. 1993;182:97–105. doi: 10.1007/BF00189377. [DOI] [PubMed] [Google Scholar]
- 86.Kuhnle A., Galuska C.E., Zlatina K., Galuska S.P. The Bovine Antimicrobial Peptide Lactoferricin Interacts with Polysialic Acid without Loss of Its Antimicrobial Activity against Escherichia coli. Animals. 2019;10:1. doi: 10.3390/ani10010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hunter H.N., Demcoe A.R., Jenssen H., Gutteberg T.J., Vogel H.J. Human lactoferricin is partially folded in aqueous solution and is better stabilized in a membrane mimetic solvent. Antimicrob. Agents Chemother. 2005;49:3387–3395. doi: 10.1128/AAC.49.8.3387-3395.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hwang P.M., Zhou N., Shan X., Arrowsmith C.H., Vogel H.J. Three-dimensional solution structure of lactoferricin B, an antimicrobial peptide derived from bovine lactoferrin. Biochemistry. 1998;37:4288–4298. doi: 10.1021/bi972323m. [DOI] [PubMed] [Google Scholar]
- 89.Vorland L.H., Ulvatne H., Andersen J., Haukland H., Rekdal O., Svendsen J.S., Gutteberg T.J. Lactoferricin of bovine origin is more active than lactoferricins of human, murine and caprine origin. Scand. J. Infect. Dis. 1998;30:513–517. doi: 10.1080/00365549850161557. [DOI] [PubMed] [Google Scholar]
- 90.Jenssen H., Andersen J.H., Uhlin-Hansen L., Gutteberg T.J., Rekdal O. Anti-HSV activity of lactoferricin analogues is only partly related to their affinity for heparan sulfate. Antivir. Res. 2004;61:101–109. doi: 10.1016/j.antiviral.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 91.Pei J., Xiong L., Chu M., Guo X., Yan P. Effect of intramolecular disulfide bond of bovine lactoferricin on its molecular structure and antibacterial activity against Trueperella pyogenes separated from cow milk with mastitis. BMC Vet. Res. 2020;16:401. doi: 10.1186/s12917-020-02620-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Andersen J.H., Osbakk S.A., Vorland L.H., Traavik T., Gutteberg T.J. Lactoferrin and cyclic lactoferricin inhibit the entry of human cytomegalovirus into human fibroblasts. Antivir. Res. 2001;51:141–149. doi: 10.1016/S0166-3542(01)00146-2. [DOI] [PubMed] [Google Scholar]
- 93.Arredondo-Beltran I.G., Ramirez-Sanchez D.A., Zazueta-Garcia J.R., Canizalez-Roman A., Angulo-Zamudio U.A., Velazquez-Roman J.A., Bolscher J.G.M., Nazmi K., Leon-Sicairos N. Antitumor activity of bovine lactoferrin and its derived peptides against HepG2 liver cancer cells and Jurkat leukemia cells. Biometals. 2023:1–17. doi: 10.1007/s10534-022-00484-4. [DOI] [PubMed] [Google Scholar]
- 94.Han F.F., Gao Y.H., Luan C., Xie Y.G., Liu Y.F., Wang Y.Z. Comparing bacterial membrane interactions and antimicrobial activity of porcine lactoferricin-derived peptides. J. Dairy Sci. 2013;96:3471–3487. doi: 10.3168/jds.2012-6104. [DOI] [PubMed] [Google Scholar]
- 95.Eliassen L.T., Berge G., Sveinbjornsson B., Svendsen J.S., Vorland L.H., Rekdal O. Evidence for a direct antitumor mechanism of action of bovine lactoferricin. Anticancer Res. 2002;22:2703–2710. [PubMed] [Google Scholar]
- 96.El-Baky N.A., Elkhawaga M.A., Abdelkhalek E.S., Sharaf M.M., Redwan E.M., Kholef H.R. De novo expression and antibacterial potential of four lactoferricin peptides in cell-free protein synthesis system. Biotechnol. Rep. 2021;29:e00583. doi: 10.1016/j.btre.2020.e00583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hwang S.A., Kruzel M.L., Actor J.K. Immunomodulatory effects of recombinant lactoferrin during MRSA infection. Int. Immunopharmacol. 2014;20:157–163. doi: 10.1016/j.intimp.2014.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Van der Kraan M.I., Groenink J., Nazmi K., Veerman E.C., Bolscher J.G., Nieuw Amerongen A.V. Lactoferrampin: A novel antimicrobial peptide in the N1-domain of bovine lactoferrin. Peptides. 2004;25:177–183. doi: 10.1016/j.peptides.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 99.Van der Kraan M.I., Nazmi K., Teeken A., Groenink J., van’t Hof W., Veerman E.C., Bolscher J.G., Nieuw Amerongen A.V. Lactoferrampin, an antimicrobial peptide of bovine lactoferrin, exerts its candidacidal activity by a cluster of positively charged residues at the C-terminus in combination with a helix-facilitating N-terminal part. Biol. Chem. 2005;386:137–142. doi: 10.1515/BC.2005.017. [DOI] [PubMed] [Google Scholar]
- 100.Haney E.F., Lau F., Vogel H.J. Solution structures and model membrane interactions of lactoferrampin, an antimicrobial peptide derived from bovine lactoferrin. Biochim. Biophys. Acta. 2007;1768:2355–2364. doi: 10.1016/j.bbamem.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 101.Haney E.F., Nazmi K., Lau F., Bolscher J.G., Vogel H.J. Novel lactoferrampin antimicrobial peptides derived from human lactoferrin. Biochimie. 2009;91:141–154. doi: 10.1016/j.biochi.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 102.Haney E.F., Nazmi K., Bolscher J.G., Vogel H.J. Structural and biophysical characterization of an antimicrobial peptide chimera comprised of lactoferricin and lactoferrampin. Biochim. Biophys. Acta. 2012;1818:762–775. doi: 10.1016/j.bbamem.2011.11.023. [DOI] [PubMed] [Google Scholar]
- 103.Bolscher J.G., Adao R., Nazmi K., van den Keybus P.A., van’t Hof W., Nieuw Amerongen A.V., Bastos M., Veerman E.C. Bactericidal activity of LFchimera is stronger and less sensitive to ionic strength than its constituent lactoferricin and lactoferrampin peptides. Biochimie. 2009;91:123–132. doi: 10.1016/j.biochi.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 104.Xu G., Xiong W., Hu Q., Zuo P., Shao B., Lan F., Lu X., Xu Y., Xiong S. Lactoferrin-derived peptides and Lactoferricin chimera inhibit virulence factor production and biofilm formation in Pseudomonas aeruginosa. J. Appl. Microbiol. 2010;109:1311–1318. doi: 10.1111/j.1365-2672.2010.04751.x. [DOI] [PubMed] [Google Scholar]
- 105.Flores-Villasenor H., Canizalez-Roman A., Reyes-Lopez M., Nazmi K., de la Garza M., Zazueta-Beltran J., Leon-Sicairos N., Bolscher J.G. Bactericidal effect of bovine lactoferrin, LFcin, LFampin and LFchimera on antibiotic-resistant Staphylococcus aureus and Escherichia coli. Biometals. 2010;23:569–578. doi: 10.1007/s10534-010-9306-4. [DOI] [PubMed] [Google Scholar]
- 106.Ruangcharoen S., Suwannarong W., Lachica M., Bolscher J.G.M., Nazmi K., Khunkitti W., Taweechaisupapong S. Killing activity of LFchimera on periodontopathic bacteria and multispecies oral biofilm formation in vitro. World J. Microbiol. Biotechnol. 2017;33:167. doi: 10.1007/s11274-017-2334-2. [DOI] [PubMed] [Google Scholar]
- 107.Aguilar-Diaz H., Canizalez-Roman A., Nepomuceno-Mejia T., Gallardo-Vera F., Hornelas-Orozco Y., Nazmi K., Bolscher J.G., Carrero J.C., Leon-Sicairos C., Leon-Sicairos N. Parasiticidal effect of synthetic bovine lactoferrin peptides on the enteric parasite Giardia intestinalis. Biochem. Cell Biol. 2017;95:82–90. doi: 10.1139/bcb-2016-0079. [DOI] [PubMed] [Google Scholar]
- 108.Lopez-Soto F., Leon-Sicairos N., Nazmi K., Bolscher J.G., de la Garza M. Microbicidal effect of the lactoferrin peptides lactoferricin17-30, lactoferrampin265-284, and lactoferrin chimera on the parasite Entamoeba histolytica. Biometals. 2010;23:563–568. doi: 10.1007/s10534-010-9295-3. [DOI] [PubMed] [Google Scholar]
- 109.Roth-Walter F. Iron-Deficiency in Atopic Diseases: Innate Immune Priming by Allergens and Siderophores. Front. Allergy. 2022;3:859922. doi: 10.3389/falgy.2022.859922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Trentini A., Maritati M., Rosta V., Cervellati C., Manfrinato M.C., Hanau S., Greco P., Bonaccorsi G., Bellini T., Contini C. Vaginal Lactoferrin Administration Decreases Oxidative Stress in the Amniotic Fluid of Pregnant Women: An Open-Label Randomized Pilot Study. Front. Med. 2020;7:555. doi: 10.3389/fmed.2020.00555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Park S.Y., Jeong A.J., Kim G.Y., Jo A., Lee J.E., Leem S.H., Yoon J.H., Ye S.K., Chung J.W. Lactoferrin Protects Human Mesenchymal Stem Cells from Oxidative Stress-Induced Senescence and Apoptosis. J. Microbiol. Biotechnol. 2017;27:1877–1884. doi: 10.4014/jmb.1707.07040. [DOI] [PubMed] [Google Scholar]
- 112.Liu H., Wu H., Zhu N., Xu Z., Wang Y., Qu Y., Wang J. Lactoferrin protects against iron dysregulation, oxidative stress, and apoptosis in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced Parkinson’s disease in mice. J. Neurochem. 2020;152:397–415. doi: 10.1111/jnc.14857. [DOI] [PubMed] [Google Scholar]
- 113.Lepanto M.S., Rosa L., Paesano R., Valenti P., Cutone A. Lactoferrin in Aseptic and Septic Inflammation. Molecules. 2019;24:1323. doi: 10.3390/molecules24071323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Weinberg E.D. Iron and infection. Microbiol. Rev. 1978;42:45–66. doi: 10.1128/mr.42.1.45-66.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang Z., Lu M.L., Chen C.L., Tong X., Li Y.H., Yang K., Lv H.T., Xu J.Y., Qin L.Q. Holo-lactoferrin: The link between ferroptosis and radiotherapy in triple-negative breast cancer. Theranostics. 2021;11:3167–3182. doi: 10.7150/thno.52028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Borregaard N., Sorensen O.E., Theilgaard-Monch K. Neutrophil granules: A library of innate immunity proteins. Trends Immunol. 2007;28:340–345. doi: 10.1016/j.it.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 117.Yang D., de la Rosa G., Tewary P., Oppenheim J.J. Alarmins link neutrophils and dendritic cells. Trends Immunol. 2009;30:531–537. doi: 10.1016/j.it.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.De la Rosa G., Yang D., Tewary P., Varadhachary A., Oppenheim J.J. Lactoferrin acts as an alarmin to promote the recruitment and activation of APCs and antigen-specific immune responses. J. Immunol. 2008;180:6868–6876. doi: 10.4049/jimmunol.180.10.6868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Muri J., Kopf M. Redox regulation of immunometabolism. Nat. Rev. Immunol. 2021;21:363–381. doi: 10.1038/s41577-020-00478-8. [DOI] [PubMed] [Google Scholar]
- 120.Forrester S.J., Kikuchi D.S., Hernandes M.S., Xu Q., Griendling K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018;122:877–902. doi: 10.1161/CIRCRESAHA.117.311401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Appelmelk B.J., An Y.Q., Geerts M., Thijs B.G., de Boer H.A., MacLaren D.M., de Graaff J., Nuijens J.H. Lactoferrin is a lipid A-binding protein. Infect. Immun. 1994;62:2628–2632. doi: 10.1128/iai.62.6.2628-2632.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Leitch E.C., Willcox M.D.P. Elucidation of the antistaphylococcal action of lactoferrin and lysozyme. J. Med. Microbiol. 1999;48:867–871. doi: 10.1099/00222615-48-9-867. [DOI] [PubMed] [Google Scholar]
- 123.Kanyshkova T.G., Semenov D.V., Buneva V.N., Nevinsky G.A. Human milk lactoferrin binds two DNA molecules with different affinities. FEBS Lett. 1999;451:235–237. doi: 10.1016/S0014-5793(99)00579-7. [DOI] [PubMed] [Google Scholar]
- 124.Britigan B.E., Lewis T.S., Waldschmidt M., McCormick M.L., Krieg A.M. Lactoferrin binds CpG-containing oligonucleotides and inhibits their immunostimulatory effects on human B cells. J. Immunol. 2001;167:2921–2928. doi: 10.4049/jimmunol.167.5.2921. [DOI] [PubMed] [Google Scholar]
- 125.Zou S., Magura C.E., Hurley W.L. Heparin-binding properties of lactoferrin and lysozyme. Comp. Biochem. Physiol. B. 1992;103:889–895. doi: 10.1016/0305-0491(92)90210-I. [DOI] [PubMed] [Google Scholar]
- 126.Zamyatina A., Heine H. Lipopolysaccharide Recognition in the Crossroads of TLR4 and Caspase-4/11 Mediated Inflammatory Pathways. Front. Immunol. 2020;11:585146. doi: 10.3389/fimmu.2020.585146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Elass-Rochard E., Legrand D., Salmon V., Roseanu A., Trif M., Tobias P.S., Mazurier J., Spik G. Lactoferrin inhibits the endotoxin interaction with CD14 by competition with the lipopolysaccharide-binding protein. Infect. Immun. 1998;66:486–491. doi: 10.1128/IAI.66.2.486-491.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mattsby-Baltzer I., Roseanu A., Motas C., Elverfors J., Engberg I., Hanson L.A. Lactoferrin or a fragment thereof inhibits the endotoxin-induced interleukin-6 response in human monocytic cells. Pediatr. Res. 1996;40:257–262. doi: 10.1203/00006450-199608000-00011. [DOI] [PubMed] [Google Scholar]
- 129.Cutone A., Rosa L., Lepanto M.S., Scotti M.J., Berlutti F., Bonaccorsi di Patti M.C., Musci G., Valenti P. Lactoferrin Efficiently Counteracts the Inflammation-Induced Changes of the Iron Homeostasis System in Macrophages. Front. Immunol. 2017;8:705. doi: 10.3389/fimmu.2017.00705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wisgrill L., Wessely I., Spittler A., Forster-Waldl E., Berger A., Sadeghi K. Human lactoferrin attenuates the proinflammatory response of neonatal monocyte-derived macrophages. Clin. Exp. Immunol. 2018;192:315–324. doi: 10.1111/cei.13108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Na Y.J., Han S.B., Kang J.S., Yoon Y.D., Park S.K., Kim H.M., Yang K.H., Joe C.O. Lactoferrin works as a new LPS-binding protein in inflammatory activation of macrophages. Int. Immunopharmacol. 2004;4:1187–1199. doi: 10.1016/j.intimp.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 132.Lonnerdal B., Du X., Jiang R. Biological activities of commercial bovine lactoferrin sources. Biochem. Cell Biol. 2021;99:35–46. doi: 10.1139/bcb-2020-0182. [DOI] [PubMed] [Google Scholar]
- 133.Kruzel M.L., Harari Y., Chen C.Y., Castro G.A. Lactoferrin protects gut mucosal integrity during endotoxemia induced by lipopolysaccharide in mice. Inflammation. 2000;24:33–44. doi: 10.1023/A:1006935908960. [DOI] [PubMed] [Google Scholar]
- 134.Kruzel M.L., Harari Y., Mailman D., Actor J.K., Zimecki M. Differential effects of prophylactic, concurrent and therapeutic lactoferrin treatment on LPS-induced inflammatory responses in mice. Clin. Exp. Immunol. 2002;130:25–31. doi: 10.1046/j.1365-2249.2002.01956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zagulski T., Lipinski P., Zagulska A., Broniek S., Jarzabek Z. Lactoferrin can protect mice against a lethal dose of Escherichia coli in experimental infection in vivo. Br. J. Exp. Pathol. 1989;70:697–704. [PMC free article] [PubMed] [Google Scholar]
- 136.Nebermann L., Dohler J.R., Perlick L. Treatment of enterogenic endotoxinemia with lactoferrin in rats. Langenbecks Arch. Surg. 2001;386:146–149. doi: 10.1007/s004230000191. [DOI] [PubMed] [Google Scholar]
- 137.Talukder M.J., Harada E. Bovine lactoferrin protects lipopolysaccharide-induced diarrhea modulating nitric oxide and prostaglandin E2 in mice. Can. J. Physiol. Pharmacol. 2007;85:200–208. doi: 10.1139/Y07-004. [DOI] [PubMed] [Google Scholar]
- 138.Actor J.K., Hwang S.A., Kruzel M.L. Lactoferrin as a natural immune modulator. Curr. Pharm. Des. 2009;15:1956–1973. doi: 10.2174/138161209788453202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hirotani Y., Ikeda K., Kato R., Myotoku M., Umeda T., Ijiri Y., Tanaka K. Protective effects of lactoferrin against intestinal mucosal damage induced by lipopolysaccharide in human intestinal Caco-2 cells. Yakugaku Zasshi. 2008;128:1363–1368. doi: 10.1248/yakushi.128.1363. [DOI] [PubMed] [Google Scholar]
- 140.Wu H., Fan L., Gao Y., Wang J., Zheng N. The Protective Effects of Iron Free Lactoferrin on Lipopolysaccharide-Induced Intestinal Inflammatory Injury via Modulating the NF-kappaB/PPAR Signaling Pathway. Foods. 2022;11:3378. doi: 10.3390/foods11213378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhao X., Xu X.X., Liu Y., Xi E.Z., An J.J., Tabys D., Liu N. The In Vitro Protective Role of Bovine Lactoferrin on Intestinal Epithelial Barrier. Molecules. 2019;24:148. doi: 10.3390/molecules24010148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Spadaro M., Caorsi C., Ceruti P., Varadhachary A., Forni G., Pericle F., Giovarelli M. Lactoferrin, a major defense protein of innate immunity, is a novel maturation factor for human dendritic cells. FASEB J. 2008;22:2747–2757. doi: 10.1096/fj.07-098038. [DOI] [PubMed] [Google Scholar]
- 143.Kubo S., Miyakawa M., Tada A., Oda H., Motobayashi H., Iwabuchi S., Tamura S., Tanaka M., Hashimoto S. Lactoferrin and its digestive peptides induce interferon-alpha production and activate plasmacytoid dendritic cells ex vivo. Biometals. 2022:1–11. doi: 10.1007/s10534-022-00436-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Puddu P., Latorre D., Carollo M., Catizone A., Ricci G., Valenti P., Gessani S. Bovine lactoferrin counteracts Toll-like receptor mediated activation signals in antigen presenting cells. PLoS ONE. 2011;6:e22504. doi: 10.1371/journal.pone.0022504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Perdijk O., van Neerven R.J.J., van den Brink E., Savelkoul H.F.J., Brugman S. Bovine Lactoferrin Modulates Dendritic Cell Differentiation and Function. Nutrients. 2018;10:848. doi: 10.3390/nu10070848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Okubo K., Kamiya M., Urano Y., Nishi H., Herter J.M., Mayadas T., Hirohama D., Suzuki K., Kawakami H., Tanaka M., et al. Lactoferrin Suppresses Neutrophil Extracellular Traps Release in Inflammation. EBioMedicine. 2016;10:204–215. doi: 10.1016/j.ebiom.2016.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yuan Y., Wu Q., Cheng G., Liu X., Liu S., Luo J., Zhang A., Bian L., Chen J., Lv J., et al. Recombinant human lactoferrin enhances the efficacy of triple therapy in mice infected with Helicobacter pylori. Int. J. Mol. Med. 2015;36:363–368. doi: 10.3892/ijmm.2015.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Welsh K.J., Hwang S.A., Boyd S., Kruzel M.L., Hunter R.L., Actor J.K. Influence of oral lactoferrin on Mycobacterium tuberculosis induced immunopathology. Tuberculosis. 2011;91:S105–S113. doi: 10.1016/j.tube.2011.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Actor J.K., Nguyen T.K.T., Wasik-Smietana A., Kruzel M.L. Modulation of TDM-induced granuloma pathology by human lactoferrin: A persistent effect in mice. Biometals. 2022:1–13. doi: 10.1007/s10534-022-00434-0. [DOI] [PubMed] [Google Scholar]
- 150.Hwang S.A., Kruzel M.L., Actor J.K. Oral recombinant human or mouse lactoferrin reduces Mycobacterium tuberculosis TDM induced granulomatous lung pathology. Biochem. Cell Biol. 2017;95:148–154. doi: 10.1139/bcb-2016-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kruzel M.L., Bacsi A., Choudhury B., Sur S., Boldogh I. Lactoferrin decreases pollen antigen-induced allergic airway inflammation in a murine model of asthma. Immunology. 2006;119:159–166. doi: 10.1111/j.1365-2567.2006.02417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wang S.B., Deng Y.Q., Ren J., Xiao B.K., Chen Z., Tao Z.Z. Lactoferrin administration into the nostril alleviates murine allergic rhinitis and its mechanisms. Scand. J. Immunol. 2013;78:507–515. doi: 10.1111/sji.12118. [DOI] [PubMed] [Google Scholar]
- 153.Lin C.C., Chuang K.C., Chen S.W., Chao Y.H., Yen C.C., Yang S.H., Chen W., Chang K.H., Chang Y.K., Chen C.M. Lactoferrin Ameliorates Ovalbumin-Induced Asthma in Mice through Reducing Dendritic-Cell-Derived Th2 Cell Responses. Int. J. Mol. Sci. 2022;23:14185. doi: 10.3390/ijms232214185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Choi G.S., Shin S.Y., Kim J.H., Lee H.Y., Palikhe N.S., Ye Y.M., Kim S.H., Park H.S. Serum lactoferrin level as a serologic biomarker for allergic rhinitis. Clin. Exp. Allergy. 2010;40:403–410. doi: 10.1111/j.1365-2222.2009.03414.x. [DOI] [PubMed] [Google Scholar]
- 155.Yanagisawa S., Nagasaki K., Chea C., Ando T., Ayuningtyas N.F., Inubushi T., Ishikado A., Imanaka H., Sugiyama E., Takahashi I., et al. Oral administration of bovine lactoferrin suppresses the progression of rheumatoid arthritis in an SKG mouse model. PLoS ONE. 2022;17:e0263254. doi: 10.1371/journal.pone.0263254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Liu N., Feng G., Zhang X., Hu Q., Sun S., Sun J., Sun Y., Wang R., Zhang Y., Wang P., et al. The Functional Role of Lactoferrin in Intestine Mucosal Immune System and Inflammatory Bowel Disease. Front. Nutr. 2021;8:759507. doi: 10.3389/fnut.2021.759507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yen C.C., Chang W.H., Tung M.C., Chen H.L., Liu H.C., Liao C.H., Lan Y.W., Chong K.Y., Yang S.H., Chen C.M. Lactoferrin Protects Hyperoxia-Induced Lung and Kidney Systemic Inflammation in an In Vivo Imaging Model of NF-kappaB/Luciferase Transgenic Mice. Mol. Imaging Biol. 2020;22:526–538. doi: 10.1007/s11307-019-01390-x. [DOI] [PubMed] [Google Scholar]
- 158.Velusamy S.K., Ganeshnarayan K., Markowitz K., Schreiner H., Furgang D., Fine D.H., Velliyagounder K. Lactoferrin knockout mice demonstrates greater susceptibility to Aggregatibacter actinomycetemcomitans-induced periodontal disease. J. Periodontol. 2013;84:1690–1701. doi: 10.1902/jop.2013.120587. [DOI] [PubMed] [Google Scholar]
- 159.Velusamy S.K., Fine D.H., Velliyagounder K. Prophylactic effect of human lactoferrin against Streptococcus mutans bacteremia in lactoferrin knockout mice. Microbes Infect. 2014;16:762–767. doi: 10.1016/j.micinf.2014.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ye Q., Zheng Y., Fan S., Qin Z., Li N., Tang A., Ai F., Zhang X., Bian Y., Dang W., et al. Lactoferrin deficiency promotes colitis-associated colorectal dysplasia in mice. PLoS ONE. 2014;9:e103298. doi: 10.1371/journal.pone.0103298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Velliyagounder K., Alsaedi W., Alabdulmohsen W., Markowitz K., Fine D.H. Oral lactoferrin protects against experimental candidiasis in mice. J. Appl. Microbiol. 2015;118:212–221. doi: 10.1111/jam.12666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Montezuma S.R., Dolezal L.D., Rageh A.A., Mar K., Jordan M., Ferrington D.A. Lactoferrin Reduces Chorioretinal Damage in the Murine Laser Model of Choroidal Neovascularization. Curr. Eye Res. 2015;40:946–953. doi: 10.3109/02713683.2014.969808. [DOI] [PubMed] [Google Scholar]
- 163.Zhang G.H., Mann D.M., Tsai C.M. Neutralization of endotoxin in vitro and in vivo by a human lactoferrin-derived peptide. Infect. Immun. 1999;67:1353–1358. doi: 10.1128/IAI.67.3.1353-1358.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Yan D., Chen D., Shen J., Xiao G., van Wijnen A.J., Im H.J. Bovine lactoferricin is anti-inflammatory and anti-catabolic in human articular cartilage and synovium. J. Cell. Physiol. 2013;228:447–456. doi: 10.1002/jcp.24151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Yan D., Kc R., Chen D., Xiao G., Im H.J. Bovine lactoferricin-induced anti-inflammation is, in part, via up-regulation of interleukin-11 by secondary activation of STAT3 in human articular cartilage. J. Biol. Chem. 2013;288:31655–31669. doi: 10.1074/jbc.M112.440420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kim J.S., Ellman M.B., Yan D., An H.S., Kc R., Li X., Chen D., Xiao G., Cs-Szabo G., Hoskin D.W., et al. Lactoferricin mediates anti-inflammatory and anti-catabolic effects via inhibition of IL-1 and LPS activity in the intervertebral disc. J. Cell. Physiol. 2013;228:1884–1896. doi: 10.1002/jcp.24350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Xie W., Song L., Wang X., Xu Y., Liu Z., Zhao D., Wang S., Fan X., Wang Z., Gao C., et al. A bovine lactoferricin-lactoferrampin-encoding Lactobacillus reuteri CO21 regulates the intestinal mucosal immunity and enhances the protection of piglets against enterotoxigenic Escherichia coli K88 challenge. Gut Microbes. 2021;13:1956281. doi: 10.1080/19490976.2021.1956281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Malone A., Clark R.F., Hoskin D.W., Power Coombs M.R. Regulation of macrophage-associated inflammatory responses by species-specific lactoferricin peptides. Front. Biosci. Landmark Ed. 2022;27:43. doi: 10.31083/j.fbl2702043. [DOI] [PubMed] [Google Scholar]
- 169.Rybarczyk J., Kieckens E., De Zutter L., Remon J.P., Vanrompay D., Cox E. Effects of lactoferrin treatment on Escherichia coli O157:H7 rectal colonization in cattle. Vet. Microbiol. 2017;202:38–46. doi: 10.1016/j.vetmic.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 170.Rybarczyk J., Khalenkow D., Kieckens E., Skirtach A.G., Cox E., Vanrompay D. Lactoferrin translocates to the nucleus of bovine rectal epithelial cells in the presence of Escherichia coli O157:H7. Vet. Res. 2019;50:75. doi: 10.1186/s13567-019-0694-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Keragala C.B., Medcalf R.L. Plasminogen: An enigmatic zymogen. Blood. 2021;137:2881–2889. doi: 10.1182/blood.2020008951. [DOI] [PubMed] [Google Scholar]
- 172.Klempner M.S., Noring R., Epstein M.P., McCloud B., Hu R., Limentani S.A., Rogers R.A. Binding of human plasminogen and urokinase-type plasminogen activator to the Lyme disease spirochete, Borrelia burgdorferi. J. Infect. Dis. 1995;171:1258–1265. doi: 10.1093/infdis/171.5.1258. [DOI] [PubMed] [Google Scholar]
- 173.Koenigs A., Hammerschmidt C., Jutras B.L., Pogoryelov D., Barthel D., Skerka C., Kugelstadt D., Wallich R., Stevenson B., Zipfel P.F., et al. BBA70 of Borrelia burgdorferi is a novel plasminogen-binding protein. J. Biol. Chem. 2013;288:25229–25243. doi: 10.1074/jbc.M112.413872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wang X., Lin X., Loy J.A., Tang J., Zhang X.C. Crystal structure of the catalytic domain of human plasmin complexed with streptokinase. Science. 1998;281:1662–1665. doi: 10.1126/science.281.5383.1662. [DOI] [PubMed] [Google Scholar]
- 175.Fuentes-Prior P. Priming of SARS-CoV-2 S protein by several membrane-bound serine proteinases could explain enhanced viral infectivity and systemic COVID-19 infection. J. Biol. Chem. 2021;296:100135. doi: 10.1074/jbc.REV120.015980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Huggins D.J. Structural analysis of experimental drugs binding to the SARS-CoV-2 target TMPRSS2. J. Mol. Graph. Model. 2020;100:107710. doi: 10.1016/j.jmgm.2020.107710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Tse L.V., Marcano V.C., Huang W., Pocwierz M.S., Whittaker G.R. Plasmin-mediated activation of pandemic H1N1 influenza virus hemagglutinin is independent of the viral neuraminidase. J. Virol. 2013;87:5161–5169. doi: 10.1128/JVI.00210-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ohradanova-Repic A., Skrabana R., Gebetsberger L., Tajti G., Barath P., Ondrovicova G., Prazenicova R., Jantova N., Hrasnova P., Stockinger H., et al. Blockade of TMPRSS2-mediated priming of SARS-CoV-2 by lactoferricin. Front. Immunol. 2022;13:958581. doi: 10.3389/fimmu.2022.958581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Krzyzowska M., Janicka M., Tomaszewska E., Ranoszek-Soliwoda K., Celichowski G., Grobelny J., Szymanski P. Lactoferrin-Conjugated Nanoparticles as New Antivirals. Pharmaceutics. 2022;14:1862. doi: 10.3390/pharmaceutics14091862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Groot F., Geijtenbeek T.B., Sanders R.W., Baldwin C.E., Sanchez-Hernandez M., Floris R., van Kooyk Y., de Jong E.C., Berkhout B. Lactoferrin prevents dendritic cell-mediated human immunodeficiency virus type 1 transmission by blocking the DC-SIGN--gp120 interaction. J. Virol. 2005;79:3009–3015. doi: 10.1128/JVI.79.5.3009-3015.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Chen J.M., Fan Y.C., Lin J.W., Chen Y.Y., Hsu W.L., Chiou S.S. Bovine Lactoferrin Inhibits Dengue Virus Infectivity by Interacting with Heparan Sulfate, Low-Density Lipoprotein Receptor, and DC-SIGN. Int. J. Mol. Sci. 2017;18:1957. doi: 10.3390/ijms18091957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Andersen J.H., Jenssen H., Sandvik K., Gutteberg T.J. Anti-HSV activity of lactoferrin and lactoferricin is dependent on the presence of heparan sulphate at the cell surface. J. Med. Virol. 2004;74:262–271. doi: 10.1002/jmv.20171. [DOI] [PubMed] [Google Scholar]
- 183.Shestakov A., Jenssen H., Nordstrom I., Eriksson K. Lactoferricin but not lactoferrin inhibit herpes simplex virus type 2 infection in mice. Antivir. Res. 2012;93:340–345. doi: 10.1016/j.antiviral.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 184.Prieto-Fernandez E., Egia-Mendikute L., Vila-Vecilla L., Bosch A., Barreira-Manrique A., Lee S.Y., Garcia-Del Rio A., Antonana-Vildosola A., Jimenez-Lasheras B., Moreno-Cugnon L., et al. Hypoxia reduces cell attachment of SARS-CoV-2 spike protein by modulating the expression of ACE2, neuropilin-1, syndecan-1 and cellular heparan sulfate. Emerg. Microbes Infect. 2021;10:1065–1076. doi: 10.1080/22221751.2021.1932607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Hu Y., Meng X., Zhang F., Xiang Y., Wang J. The in vitro antiviral activity of lactoferrin against common human coronaviruses and SARS-CoV-2 is mediated by targeting the heparan sulfate co-receptor. Emerg. Microbes Infect. 2021;10:317–330. doi: 10.1080/22221751.2021.1888660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Lang J., Yang N., Deng J., Liu K., Yang P., Zhang G., Jiang C. Inhibition of SARS pseudovirus cell entry by lactoferrin binding to heparan sulfate proteoglycans. PLoS ONE. 2011;6:e23710. doi: 10.1371/journal.pone.0023710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Beljaars L., van der Strate B.W., Bakker H.I., Reker-Smit C., van Loenen-Weemaes A.M., Wiegmans F.C., Harmsen M.C., Molema G., Meijer D.K. Inhibition of cytomegalovirus infection by lactoferrin in vitro and in vivo. Antivir. Res. 2004;63:197–208. doi: 10.1016/j.antiviral.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 188.Carvalho C.A.M., Casseb S.M.M., Goncalves R.B., Silva E.V.P., Gomes A.M.O., Vasconcelos P.F.C. Bovine lactoferrin activity against Chikungunya and Zika viruses. J. Gen. Virol. 2017;98:1749–1754. doi: 10.1099/jgv.0.000849. [DOI] [PubMed] [Google Scholar]
- 189.Cutone A., Rosa L., Bonaccorsi di Patti M.C., Iacovelli F., Conte M.P., Ianiro G., Romeo A., Campione E., Bianchi L., Valenti P., et al. Lactoferrin Binding to SARS-CoV-2 Spike Glycoprotein Blocks Pseudoviral Entry and Relieves Iron Protein Dysregulation in Several In Vitro Models. Pharmaceutics. 2022;14:2111. doi: 10.3390/pharmaceutics14102111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ikeda M., Sugiyama K., Tanaka T., Tanaka K., Sekihara H., Shimotohno K., Kato N. Lactoferrin markedly inhibits hepatitis C virus infection in cultured human hepatocytes. Biochem. Biophys. Res. Commun. 1998;245:549–553. doi: 10.1006/bbrc.1998.8481. [DOI] [PubMed] [Google Scholar]
- 191.Nozaki A., Ikeda M., Naganuma A., Nakamura T., Inudoh M., Tanaka K., Kato N. Identification of a lactoferrin-derived peptide possessing binding activity to hepatitis C virus E2 envelope protein. J. Biol. Chem. 2003;278:10162–10173. doi: 10.1074/jbc.M207879200. [DOI] [PubMed] [Google Scholar]
- 192.Naidu S.A.G., Clemens R.A., Pressman P., Zaigham M., Davies K.J.A., Naidu A.S. COVID-19 during Pregnancy and Postpartum: Antiviral Spectrum of Maternal Lactoferrin in Fetal and Neonatal Defense. J. Diet. Suppl. 2020;19:1–37. doi: 10.1080/19390211.2020.1834047. [DOI] [PubMed] [Google Scholar]
- 193.Campione E., Lanna C., Cosio T., Rosa L., Conte M.P., Iacovelli F., Romeo A., Falconi M., Del Vecchio C., Franchin E., et al. Lactoferrin Against SARS-CoV-2: In Vitro and In Silico Evidences. Front. Pharmacol. 2021;12:666600. doi: 10.3389/fphar.2021.666600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Patil D., Chen S., Fogliano V., Madadlou A. Hydrolysis improves the inhibition efficacy of bovine lactoferrin against infection by SARS-CoV-2 pseudovirus. Int. Dairy J. 2023;137:105488. doi: 10.1016/j.idairyj.2022.105488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Mirabelli C., Wotring J.W., Zhang C.J., McCarty S.M., Fursmidt R., Pretto C.D., Qiao Y., Zhang Y., Frum T., Kadambi N.S., et al. Morphological cell profiling of SARS-CoV-2 infection identifies drug repurposing candidates for COVID-19. Proc. Natl. Acad. Sci. USA. 2021;118:e2105815118. doi: 10.1073/pnas.2105815118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Lai X., Yu Y., Xian W., Ye F., Ju X., Luo Y., Dong H., Zhou Y.H., Tan W., Zhuang H., et al. Identified human breast milk compositions effectively inhibit SARS-CoV-2 and variants infection and replication. iScience. 2022;25:104136. doi: 10.1016/j.isci.2022.104136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.He S.T., Qin H., Guan L., Liu K., Hong B., Zhang X., Lou F., Li M., Lin W., Chen Y., et al. Bovine lactoferrin inhibits SARS-CoV-2 and SARS-CoV-1 by targeting the RdRp complex and alleviates viral infection in the hamster model. J. Med. Virol. 2023;95:e28281. doi: 10.1002/jmv.28281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Naidu S.A.G., Clemens R.A., Naidu A.S. SARS-CoV-2 Infection Dysregulates Host Iron (Fe)-Redox Homeostasis (Fe-R-H): Role of Fe-Redox Regulators, Ferroptosis Inhibitors, Anticoagulants, and Iron-Chelators in COVID-19 Control. J. Diet. Suppl. 2022;20:1–60. doi: 10.1080/19390211.2022.2075072. [DOI] [PubMed] [Google Scholar]
- 199.Zhao W., Li X., Yu Z., Wu S., Ding L., Liu J. Identification of lactoferrin-derived peptides as potential inhibitors against the main protease of SARS-CoV-2. Lebensm. Wiss. Technol. 2022;154:112684. doi: 10.1016/j.lwt.2021.112684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Wotring J.W., Fursmidt R., Ward L., Sexton J.Z. Evaluating the in vitro efficacy of bovine lactoferrin products against SARS-CoV-2 variants of concern. J. Dairy Sci. 2022;105:2791–2802. doi: 10.3168/jds.2021-21247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Avalos-Gomez C., Ramirez-Rico G., Ruiz-Mazon L., Sicairos N.L., Serrano-Luna J., de la Garza M. Lactoferrin: An Effective Weapon in the Battle Against Bacterial Infections. Curr. Pharm. Des. 2022;28:3243–3260. doi: 10.2174/1381612829666221025153216. [DOI] [PubMed] [Google Scholar]
- 202.Sallmann F.R., Baveye-Descamps S., Pattus F., Salmon V., Branza N., Spik G., Legrand D. Porins OmpC and PhoE of Escherichia coli as specific cell-surface targets of human lactoferrin. Binding characteristics and biological effects. J. Biol. Chem. 1999;274:16107–16114. doi: 10.1074/jbc.274.23.16107. [DOI] [PubMed] [Google Scholar]
- 203.Ellison R.T., 3rd, Giehl T.J., LaForce F.M. Damage of the outer membrane of enteric gram-negative bacteria by lactoferrin and transferrin. Infect. Immun. 1988;56:2774–2781. doi: 10.1128/iai.56.11.2774-2781.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Ellison R.T., 3rd, Giehl T.J. Killing of gram-negative bacteria by lactoferrin and lysozyme. J. Clin. Investig. 1991;88:1080–1091. doi: 10.1172/JCI115407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Singh P.K., Parsek M.R., Greenberg E.P., Welsh M.J. A component of innate immunity prevents bacterial biofilm development. Nature. 2002;417:552–555. doi: 10.1038/417552a. [DOI] [PubMed] [Google Scholar]
- 206.Dashper S.G., Pan Y., Veith P.D., Chen Y.Y., Toh E.C., Liu S.W., Cross K.J., Reynolds E.C. Lactoferrin inhibits Porphyromonas gingivalis proteinases and has sustained biofilm inhibitory activity. Antimicrob. Agents Chemother. 2012;56:1548–1556. doi: 10.1128/AAC.05100-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Ochoa T.J., Clearly T.G. Lactoferrin disruption of bacterial type III secretion systems. Biometals. 2004;17:257–260. doi: 10.1023/B:BIOM.0000027701.12965.d4. [DOI] [PubMed] [Google Scholar]
- 208.Dierick M., Ongena R., Vanrompay D., Devriendt B., Cox E. Lactoferrin Decreases Enterotoxigenic Escherichia coli-Induced Fluid Secretion and Bacterial Adhesion in the Porcine Small Intestine. Pharmaceutics. 2022;14:1778. doi: 10.3390/pharmaceutics14091778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Silva T., Magalhaes B., Maia S., Gomes P., Nazmi K., Bolscher J.G., Rodrigues P.N., Bastos M., Gomes M.S. Killing of Mycobacterium avium by lactoferricin peptides: Improved activity of arginine- and D-amino-acid-containing molecules. Antimicrob. Agents Chemother. 2014;58:3461–3467. doi: 10.1128/AAC.02728-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Hossain F., Moghal M.M.R., Islam M.Z., Moniruzzaman M., Yamazaki M. Membrane potential is vital for rapid permeabilization of plasma membranes and lipid bilayers by the antimicrobial peptide lactoferricin B. J. Biol. Chem. 2019;294:10449–10462. doi: 10.1074/jbc.RA119.007762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Aguilera O., Ostolaza H., Quiros L.M., Fierro J.F. Permeabilizing action of an antimicrobial lactoferricin-derived peptide on bacterial and artificial membranes. FEBS Lett. 1999;462:273–277. doi: 10.1016/S0014-5793(99)01545-8. [DOI] [PubMed] [Google Scholar]
- 212.Farnaud S., Spiller C., Moriarty L.C., Patel A., Gant V., Odell E.W., Evans R.W. Interactions of lactoferricin-derived peptides with LPS and antimicrobial activity. FEMS Microbiol. Lett. 2004;233:193–199. doi: 10.1111/j.1574-6968.2004.tb09482.x. [DOI] [PubMed] [Google Scholar]
- 213.Hossain F., Dohra H., Yamazaki M. Effect of membrane potential on entry of lactoferricin B-derived 6-residue antimicrobial peptide into single Escherichia coli cells and lipid vesicles. J. Bacteriol. 2021;203:e00021. doi: 10.1128/JB.00021-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Wuersching S.N., Huth K.C., Hickel R., Kollmuss M. Targeting antibiotic tolerance in anaerobic biofilms associated with oral diseases: Human antimicrobial peptides LL-37 and lactoferricin enhance the antibiotic efficacy of amoxicillin, clindamycin and metronidazole. Anaerobe. 2021;71:102439. doi: 10.1016/j.anaerobe.2021.102439. [DOI] [PubMed] [Google Scholar]
- 215.Kim S., Hwang J.S., Lee D.G. Lactoferricin B like peptide triggers mitochondrial disruption-mediated apoptosis by inhibiting respiration under nitric oxide accumulation in Candida albicans. IUBMB Life. 2020;72:1515–1527. doi: 10.1002/iub.2284. [DOI] [PubMed] [Google Scholar]
- 216.Frontera L.S., Moyano S., Quassollo G., Lanfredi-Rangel A., Ropolo A.S., Touz M.C. Lactoferrin and lactoferricin endocytosis halt Giardia cell growth and prevent infective cyst production. Sci. Rep. 2018;8:18020. doi: 10.1038/s41598-018-36563-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Haversen L.A., Engberg I., Baltzer L., Dolphin G., Hanson L.A., Mattsby-Baltzer I. Human lactoferrin and peptides derived from a surface-exposed helical region reduce experimental Escherichia coli urinary tract infection in mice. Infect. Immun. 2000;68:5816–5823. doi: 10.1128/IAI.68.10.5816-5823.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Fais R., Rizzato C., Franconi I., Tavanti A., Lupetti A. Synergistic Activity of the Human Lactoferricin-Derived Peptide hLF1-11 in Combination with Caspofungin against Candida Species. Microbiol. Spectr. 2022;10:e0124022. doi: 10.1128/spectrum.01240-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Sanchez-Gomez S., Ferrer-Espada R., Stewart P.S., Pitts B., Lohner K., Martinez de Tejada G. Antimicrobial activity of synthetic cationic peptides and lipopeptides derived from human lactoferricin against Pseudomonas aeruginosa planktonic cultures and biofilms. BMC Microbiol. 2015;15:137. doi: 10.1186/s12866-015-0473-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Morici P., Fais R., Rizzato C., Tavanti A., Lupetti A. Inhibition of Candida albicans Biofilm Formation by the Synthetic Lactoferricin Derived Peptide hLF1-11. PLoS ONE. 2016;11:e0167470. doi: 10.1371/journal.pone.0167470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Harris M.R., Coote P.J. Combination of caspofungin or anidulafungin with antimicrobial peptides results in potent synergistic killing of Candida albicans and Candida glabrata in vitro. Int. J. Antimicrob. Agents. 2010;35:347–356. doi: 10.1016/j.ijantimicag.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 222.Faber C., Stallmann H.P., Lyaruu D.M., Joosten U., von Eiff C., van Nieuw Amerongen A., Wuisman P.I. Comparable efficacies of the antimicrobial peptide human lactoferrin 1-11 and gentamicin in a chronic methicillin-resistant Staphylococcus aureus osteomyelitis model. Antimicrob. Agents Chemother. 2005;49:2438–2444. doi: 10.1128/AAC.49.6.2438-2444.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Shaper M., Hollingshead S.K., Benjamin W.H., Jr., Briles D.E. PspA protects Streptococcus pneumoniae from killing by apolactoferrin, and antibody to PspA enhances killing of pneumococci by apolactoferrin [corrected] Infect. Immun. 2004;72:5031–5040. doi: 10.1128/IAI.72.9.5031-5040.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Yadav R., Noinaj N., Ostan N., Moraes T., Stoudenmire J., Maurakis S., Cornelissen C.N. Structural Basis for Evasion of Nutritional Immunity by the Pathogenic Neisseriae. Front. Microbiol. 2019;10:2981. doi: 10.3389/fmicb.2019.02981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Ostan N.K., Yu R.H., Ng D., Lai C.C., Pogoutse A.K., Sarpe V., Hepburn M., Sheff J., Raval S., Schriemer D.C., et al. Lactoferrin binding protein B-a bi-functional bacterial receptor protein. PLoS Pathog. 2017;13:e1006244. doi: 10.1371/journal.ppat.1006244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Yadav R., Govindan S., Daczkowski C., Mesecar A., Chakravarthy S., Noinaj N. Structural insight into the dual function of LbpB in mediating Neisserial pathogenesis. eLife. 2021;10:e71683. doi: 10.7554/eLife.71683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Morgenthau A., Livingstone M., Adamiak P., Schryvers A.B. The role of lactoferrin binding protein B in mediating protection against human lactoferricin. Biochem. Cell Biol. 2012;90:417–423. doi: 10.1139/o11-074. [DOI] [PubMed] [Google Scholar]
- 228.Schryvers A.B. Targeting bacterial transferrin and lactoferrin receptors for vaccines. Trends Microbiol. 2022;30:820–830. doi: 10.1016/j.tim.2022.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Deng M., Zhang W., Tang H., Ye Q., Liao Q., Zhou Y., Wu M., Xiong W., Zheng Y., Guo X., et al. Lactotransferrin acts as a tumor suppressor in nasopharyngeal carcinoma by repressing AKT through multiple mechanisms. Oncogene. 2013;32:4273–4283. doi: 10.1038/onc.2012.434. [DOI] [PubMed] [Google Scholar]
- 230.Wei L., Zhang X., Wang J., Ye Q., Zheng X., Peng Q., Zheng Y., Liu P., Zhang X., Li Z., et al. Lactoferrin deficiency induces a pro-metastatic tumor microenvironment through recruiting myeloid-derived suppressor cells in mice. Oncogene. 2020;39:122–135. doi: 10.1038/s41388-019-0970-8. [DOI] [PubMed] [Google Scholar]
- 231.Xiao Y., Monitto C.L., Minhas K.M., Sidransky D. Lactoferrin down-regulates G1 cyclin-dependent kinases during growth arrest of head and neck cancer cells. Clin. Cancer Res. 2004;10:8683–8686. doi: 10.1158/1078-0432.CCR-04-0988. [DOI] [PubMed] [Google Scholar]
- 232.Damiens E., El Yazidi I., Mazurier J., Duthille I., Spik G., Boilly-Marer Y. Lactoferrin inhibits G1 cyclin-dependent kinases during growth arrest of human breast carcinoma cells. J. Cell. Biochem. 1999;74:486–498. doi: 10.1002/(SICI)1097-4644(19990901)74:3<486::AID-JCB16>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 233.Iglesias-Figueroa B.F., Siqueiros-Cendon T.S., Gutierrez D.A., Aguilera R.J., Espinoza-Sanchez E.A., Arevalo-Gallegos S., Varela-Ramirez A., Rascon-Cruz Q. Recombinant human lactoferrin induces apoptosis, disruption of F-actin structure and cell cycle arrest with selective cytotoxicity on human triple negative breast cancer cells. Apoptosis. 2019;24:562–577. doi: 10.1007/s10495-019-01539-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Zhou Y., Zeng Z., Zhang W., Xiong W., Wu M., Tan Y., Yi W., Xiao L., Li X., Huang C., et al. Lactotransferrin: A candidate tumor suppressor-Deficient expression in human nasopharyngeal carcinoma and inhibition of NPC cell proliferation by modulating the mitogen-activated protein kinase pathway. Int. J. Cancer. 2008;123:2065–2072. doi: 10.1002/ijc.23727. [DOI] [PubMed] [Google Scholar]
- 235.Fujita K., Matsuda E., Sekine K., Iigo M., Tsuda H. Lactoferrin enhances Fas expression and apoptosis in the colon mucosa of azoxymethane-treated rats. Carcinogenesis. 2004;25:1961–1966. doi: 10.1093/carcin/bgh205. [DOI] [PubMed] [Google Scholar]
- 236.Rocha V.P., Campos S.P.C., Barros C.A., Trindade P., Souza L.R.Q., Silva T.G., Gimba E.R.P., Teodoro A.J., Goncalves R.B. Bovine Lactoferrin Induces Cell Death in Human Prostate Cancer Cells. Oxidative Med. Cell. Longev. 2022;2022:2187696. doi: 10.1155/2022/2187696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Luzi C., Brisdelli F., Iorio R., Bozzi A., Carnicelli V., Di Giulio A., Lizzi A.R. Apoptotic effects of bovine apo-lactoferrin on HeLa tumor cells. Cell Biochem. Funct. 2017;35:33–41. doi: 10.1002/cbf.3242. [DOI] [PubMed] [Google Scholar]
- 238.Shimamura M., Yamamoto Y., Ashino H., Oikawa T., Hazato T., Tsuda H., Iigo M. Bovine lactoferrin inhibits tumor-induced angiogenesis. Int. J. Cancer. 2004;111:111–116. doi: 10.1002/ijc.20187. [DOI] [PubMed] [Google Scholar]
- 239.Ayuningtyas N.F., Chea C., Ando T., Saninggar K.E., Tanimoto K., Inubushi T., Maishi N., Hida K., Shindoh M., Miyauchi M., et al. Bovine Lactoferrin Suppresses Tumor Angiogenesis through NF-kappaB Pathway Inhibition by Binding to TRAF6. Pharmaceutics. 2023;15:165. doi: 10.3390/pharmaceutics15010165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Li H.Y., Li M., Luo C.C., Wang J.Q., Zheng N. Lactoferrin Exerts Antitumor Effects by Inhibiting Angiogenesis in a HT29 Human Colon Tumor Model. J. Agric. Food Chem. 2017;65:10464–10472. doi: 10.1021/acs.jafc.7b03390. [DOI] [PubMed] [Google Scholar]
- 241.Cutone A., Colella B., Pagliaro A., Rosa L., Lepanto M.S., Bonaccorsi di Patti M.C., Valenti P., Di Bartolomeo S., Musci G. Native and iron-saturated bovine lactoferrin differently hinder migration in a model of human glioblastoma by reverting epithelial-to-mesenchymal transition-like process and inhibiting interleukin-6/STAT3 axis. Cell. Signal. 2020;65:109461. doi: 10.1016/j.cellsig.2019.109461. [DOI] [PubMed] [Google Scholar]
- 242.Costantini E., Di Nicola M., Marchioni M., Aielli L., Reale M., Ships L. Effects of Curcumin and Lactoferrin to Inhibit the Growth and Migration of Prostatic Cancer Cells. Int. J. Environ. Res. Public Health. 2022;19:16193. doi: 10.3390/ijerph192316193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Chea C., Miyauchi M., Inubushi T., Okamoto K., Haing S., Nguyen P.T., Imanaka H., Takata T. Bovine lactoferrin reverses programming of epithelial-to-mesenchymal transition to mesenchymal-to-epithelial transition in oral squamous cell carcinoma. Biochem. Biophys. Res. Commun. 2018;507:142–147. doi: 10.1016/j.bbrc.2018.10.193. [DOI] [PubMed] [Google Scholar]
- 244.Wolf J.S., Li G., Varadhachary A., Petrak K., Schneyer M., Li D., Ongkasuwan J., Zhang X., Taylor R.J., Strome S.E., et al. Oral lactoferrin results in T cell-dependent tumor inhibition of head and neck squamous cell carcinoma in vivo. Clin. Cancer Res. 2007;13:1601–1610. doi: 10.1158/1078-0432.CCR-06-2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Spadaro M., Curcio C., Varadhachary A., Cavallo F., Engelmayer J., Blezinger P., Pericle F., Forni G. Requirement for IFN-gamma, CD8+ T lymphocytes, and NKT cells in talactoferrin-induced inhibition of neu+ tumors. Cancer Res. 2007;67:6425–6432. doi: 10.1158/0008-5472.CAN-06-4080. [DOI] [PubMed] [Google Scholar]
- 246.Shi H., Li W. Inhibitory effects of human lactoferrin on U14 cervical carcinoma through upregulation of the immune response. Oncol. Lett. 2014;7:820–826. doi: 10.3892/ol.2013.1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Varadhachary A., Wolf J.S., Petrak K., O’Malley B.W., Jr., Spadaro M., Curcio C., Forni G., Pericle F. Oral lactoferrin inhibits growth of established tumors and potentiates conventional chemotherapy. Int. J. Cancer. 2004;111:398–403. doi: 10.1002/ijc.20271. [DOI] [PubMed] [Google Scholar]
- 248.Sun X., Jiang R., Przepiorski A., Reddy S., Palmano K.P., Krissansen G.W. “Iron-saturated” bovine lactoferrin improves the chemotherapeutic effects of tamoxifen in the treatment of basal-like breast cancer in mice. BMC Cancer. 2012;12:591. doi: 10.1186/1471-2407-12-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Kondapi A.K. Targeting cancer with lactoferrin nanoparticles: Recent advances. Nanomedicine. 2020;15:2071–2083. doi: 10.2217/nnm-2020-0090. [DOI] [PubMed] [Google Scholar]
- 250.Shankaranarayanan J.S., Kanwar J.R., Al-Juhaishi A.J., Kanwar R.K. Doxorubicin Conjugated to Immunomodulatory Anticancer Lactoferrin Displays Improved Cytotoxicity Overcoming Prostate Cancer Chemo resistance and Inhibits Tumour Development in TRAMP Mice. Sci. Rep. 2016;6:32062. doi: 10.1038/srep32062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Pan S., Weng H., Hu G., Wang S., Zhao T., Yao X., Liao L., Zhu X., Ge Y. Lactoferrin may inhibit the development of cancer via its immunostimulatory and immunomodulatory activities (Review) Int. J. Oncol. 2021;59:1–11. doi: 10.3892/ijo.2021.5265. [DOI] [PubMed] [Google Scholar]
- 252.Riedl S., Leber R., Rinner B., Schaider H., Lohner K., Zweytick D. Human lactoferricin derived di-peptides deploying loop structures induce apoptosis specifically in cancer cells through targeting membranous phosphatidylserine. Biochim. Biophys. Acta. 2015;1848:2918–2931. doi: 10.1016/j.bbamem.2015.07.018. [DOI] [PubMed] [Google Scholar]
- 253.Yoo Y.C., Watanabe R., Koike Y., Mitobe M., Shimazaki K., Watanabe S., Azuma I. Apoptosis in human leukemic cells induced by lactoferricin, a bovine milk protein-derived peptide: Involvement of reactive oxygen species. Biochem. Biophys. Res. Commun. 1997;237:624–628. doi: 10.1006/bbrc.1997.7199. [DOI] [PubMed] [Google Scholar]
- 254.Jiang R., Lonnerdal B. Bovine lactoferrin and lactoferricin exert antitumor activities on human colorectal cancer cells (HT-29) by activating various signaling pathways. Biochem. Cell Biol. 2017;95:99–109. doi: 10.1139/bcb-2016-0094. [DOI] [PubMed] [Google Scholar]
- 255.Pan W.R., Chen P.W., Chen Y.L., Hsu H.C., Lin C.C., Chen W.J. Bovine lactoferricin B induces apoptosis of human gastric cancer cell line AGS by inhibition of autophagy at a late stage. J. Dairy Sci. 2013;96:7511–7520. doi: 10.3168/jds.2013-7285. [DOI] [PubMed] [Google Scholar]
- 256.Zhang P., Liu J., Li W., Li S., Han X. Lactoferricin B reverses cisplatin resistance in head and neck squamous cell carcinoma cells through targeting PD-L1. Cancer Med. 2018;7:3178–3187. doi: 10.1002/cam4.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA); Scientific Opinion on bovine lactoferrin. EFSA J. 2012;10:2701. doi: 10.2903/j.efsa.2012.2701. [DOI] [Google Scholar]
- 258.Zea-Vera A., Ochoa T.J. Challenges in the diagnosis and management of neonatal sepsis. J. Trop. Pediatr. 2015;61:1–13. doi: 10.1093/tropej/fmu079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Manzoni P., Rinaldi M., Cattani S., Pugni L., Romeo M.G., Messner H., Stolfi I., Decembrino L., Laforgia N., Vagnarelli F., et al. Bovine lactoferrin supplementation for prevention of late-onset sepsis in very low-birth-weight neonates: A randomized trial. JAMA. 2009;302:1421–1428. doi: 10.1001/jama.2009.1403. [DOI] [PubMed] [Google Scholar]
- 260.Ochoa T., Loli S., Mendoza K., Carcamo C., Bellomo S., Cam L., Castaneda A., Campos M., Jacobs J., Cossey V., et al. Effect of bovine lactoferrin on prevention of late-onset sepsis in infants <1500 g: A pooled analysis of individual patient data from two randomized controlled trials. Biochem. Cell Biol. 2021;99:14–19. doi: 10.1139/bcb-2020-0046. [DOI] [PubMed] [Google Scholar]
- 261.Telang S. Lactoferrin: A Critical Player in Neonatal Host Defense. Nutrients. 2018;10:1228. doi: 10.3390/nu10091228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Akin I.M., Atasay B., Dogu F., Okulu E., Arsan S., Karatas H.D., Ikinciogullari A., Turmen T. Oral lactoferrin to prevent nosocomial sepsis and necrotizing enterocolitis of premature neonates and effect on T-regulatory cells. Am. J. Perinatol. 2014;31:1111–1120. doi: 10.1055/s-0034-1371704. [DOI] [PubMed] [Google Scholar]
- 263.Griffiths J., Jenkins P., Vargova M., Bowler U., Juszczak E., King A., Linsell L., Murray D., Partlett C., Patel M., et al. Enteral lactoferrin supplementation for very preterm infants: A randomised placebo-controlled trial. Lancet. 2019;393:423–433. doi: 10.1016/S0140-6736(18)32221-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Bjormsjo M., Hernell O., Lonnerdal B., Berglund S.K. Immunological Effects of Adding Bovine Lactoferrin and Reducing Iron in Infant Formula: A Randomized Controlled Trial. J. Pediatr. Gastroenterol. Nutr. 2022;74:e65–e72. doi: 10.1097/MPG.0000000000003367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Ochoa T.J., Chea-Woo E., Baiocchi N., Pecho I., Campos M., Prada A., Valdiviezo G., Lluque A., Lai D., Cleary T.G. Randomized double-blind controlled trial of bovine lactoferrin for prevention of diarrhea in children. J. Pediatr. 2013;162:349–356. doi: 10.1016/j.jpeds.2012.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Zavaleta N., Figueroa D., Rivera J., Sanchez J., Alfaro S., Lonnerdal B. Efficacy of rice-based oral rehydration solution containing recombinant human lactoferrin and lysozyme in Peruvian children with acute diarrhea. J. Pediatr. Gastroenterol. Nutr. 2007;44:258–264. doi: 10.1097/MPG.0b013e31802c41b7. [DOI] [PubMed] [Google Scholar]
- 267.Laffan A.M., McKenzie R., Forti J., Conklin D., Marcinko R., Shrestha R., Bellantoni M., Greenough W.B., 3rd Lactoferrin for the prevention of post-antibiotic diarrhoea. J. Health Popul. Nutr. 2011;29:547–551. doi: 10.3329/jhpn.v29i6.9889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Hablass F.H., Lashen S.A., Alsayed E.A. Efficacy of Lactoferrin with Standard Triple Therapy or Sequential Therapy for Helicobacter pylori Eradication: A Randomized Controlled Trial. Turk. J. Gastroenterol. 2021;32:742–749. doi: 10.5152/tjg.2021.20923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Nakano M., Yoshida A., Wakabayashi H., Tanaka M., Yamauchi K., Abe F., Masuda Y. Effect of tablets containing lactoferrin and lactoperoxidase on gingival health in adults: A randomized, double-blind, placebo-controlled clinical trial. J. Periodontal Res. 2019;54:702–708. doi: 10.1111/jre.12679. [DOI] [PubMed] [Google Scholar]
- 270.Ali A.S., Hasan S.S., Kow C.S., Merchant H.A. Lactoferrin reduces the risk of respiratory tract infections: A meta-analysis of randomized controlled trials. Clin. Nutr. ESPEN. 2021;45:26–32. doi: 10.1016/j.clnesp.2021.08.019. [DOI] [PubMed] [Google Scholar]
- 271.Kozu T., Iinuma G., Ohashi Y., Saito Y., Akasu T., Saito D., Alexander D.B., Iigo M., Kakizoe T., Tsuda H. Effect of orally administered bovine lactoferrin on the growth of adenomatous colorectal polyps in a randomized, placebo-controlled clinical trial. Cancer Prev Res. 2009;2:975–983. doi: 10.1158/1940-6207.CAPR-08-0208. [DOI] [PubMed] [Google Scholar]
- 272.Iigo M., Alexander D.B., Xu J., Futakuchi M., Suzui M., Kozu T., Akasu T., Saito D., Kakizoe T., Yamauchi K., et al. Inhibition of intestinal polyp growth by oral ingestion of bovine lactoferrin and immune cells in the large intestine. Biometals. 2014;27:1017–1029. doi: 10.1007/s10534-014-9747-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.D’Amico F., Decembrino N., Muratore E., Turroni S., Muggeo P., Mura R., Perruccio K., Vitale V., Zecca M., Prete A., et al. Oral Lactoferrin Supplementation during Induction Chemotherapy Promotes Gut Microbiome Eubiosis in Pediatric Patients with Hematologic Malignancies. Pharmaceutics. 2022;14:1705. doi: 10.3390/pharmaceutics14081705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Lesser G.J., Irby M.B., Taylor R.C., Snavely A., Case D., Wang A., Dietrich A., Duncan S. Lactoferrin supplementation for taste and smell abnormalities among patients receiving cancer chemotherapy. Support. Care Cancer. 2022;30:2017–2025. doi: 10.1007/s00520-021-06609-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Paesano R., Berlutti F., Pietropaoli M., Goolsbee W., Pacifici E., Valenti P. Lactoferrin efficacy versus ferrous sulfate in curing iron disorders in pregnant and non-pregnant women. Int. J. Immunopathol. Pharmacol. 2010;23:577–587. doi: 10.1177/039463201002300220. [DOI] [PubMed] [Google Scholar]
- 276.El Amrousy D., El-Afify D., Elsawy A., Elsheikh M., Donia A., Nassar M. Lactoferrin for iron-deficiency anemia in children with inflammatory bowel disease: A clinical trial. Pediatr. Res. 2022;92:762–766. doi: 10.1038/s41390-022-02136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Rosa L., Tripepi G., Naldi E., Aimati M., Santangeli S., Venditto F., Caldarelli M., Valenti P. Ambulatory COVID-19 Patients Treated with Lactoferrin as a Supplementary Antiviral Agent: A Preliminary Study. J. Clin. Med. 2021;10:4276. doi: 10.3390/jcm10184276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Campione E., Lanna C., Cosio T., Rosa L., Conte M.P., Iacovelli F., Romeo A., Falconi M., Del Vecchio C., Franchin E., et al. Lactoferrin as Antiviral Treatment in COVID-19 Management: Preliminary Evidence. Int. J. Environ. Res. Public Health. 2021;18:10985. doi: 10.3390/ijerph182010985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Navarro R., Paredes J.L., Tucto L., Medina C., Angles-Yanqui E., Nario J.C., Ruiz-Cabrejos J., Quintana J.L., Turpo-Espinoza K., Mejia-Cordero F., et al. Bovine lactoferrin for the prevention of COVID-19 infection in health care personnel: A double-blinded randomized clinical trial (LF-COVID) Biometals. 2022;7:1–10. doi: 10.1007/s10534-022-00477-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Algahtani F.D., Elabbasy M.T., Samak M.A., Adeboye A.A., Yusuf R.A., Ghoniem M.E. The Prospect of Lactoferrin Use as Adjunctive Agent in Management of SARS-CoV-2 Patients: A Randomized Pilot Study. Medicina. 2021;57:842. doi: 10.3390/medicina57080842. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available in this article.