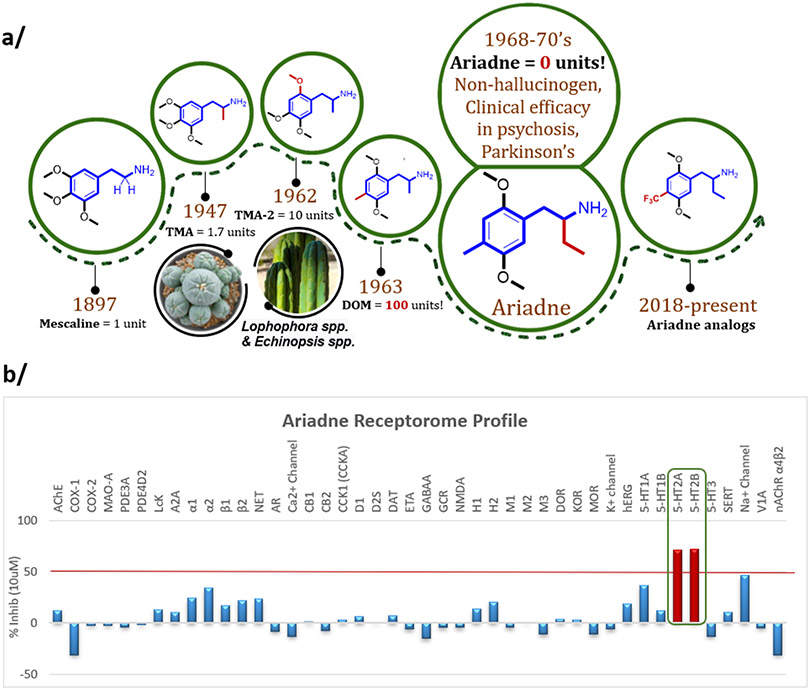
Figure 1.
Ariadne is a non-hallucinogenic analog of phenylalkylamine psychedelics with remarkable efficacy signals in clinical studies. (a) The mescaline molecular lineage of Ariadne, which contains an ethyl group in the alpha-position to the amine. The units represent an approximate measure of psycho-activity in humans relative to mescaline based on subjective reports after per oral administration.7 In contrast to DOM, a potent psychedelic, Ariadne showed no hallucinogenic effects at doses that induced notable clinical effects. The present work introduces novel analogs of Ariadne. (b) Broad receptor screen (SafetyScreen44, Eurofins-Panlabs) identified the serotonin 5-HT2A and 2B receptors as the only hits above the set threshold (more than 50% displacement of standard radioligands at 10 uM concentration of (rac)-Ariadne). TMA = 3,4,5-trimethoxyamphetamine; TMA-2 = 2,4,5-trimethoxyamphetamine; DOM = 2,5-dimethoxy-4-methyl-amphetamine.