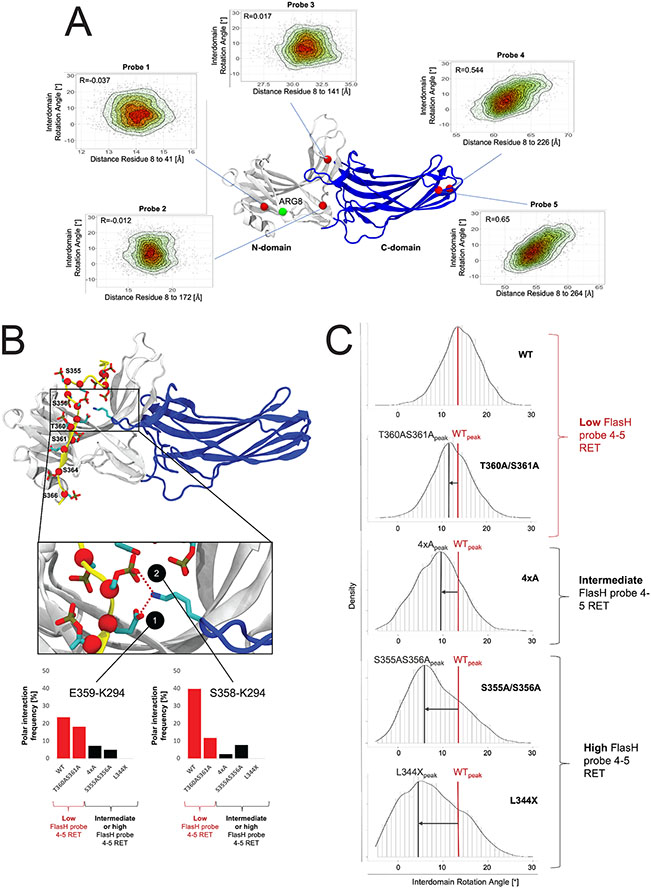
Figure 4: Impact of the phosphorylation pattern on β-arrestin 2 conformational dynamics.
(A) Heat scatter plots of the interdomain rotation angle of β-arrestin 2 (a measure of activation) and probe distances (probe 1 to 5) to the RLuc domain. The interdomain rotation angles and corresponding probe distances have been computed for simulation frames sampling β-arrestin 2 inactivation. A structural model of the construct used in the FlAsH BRET conformational assay (N-domain in grey and the C-domain in blue) highlights the positions of probes 1-5 (red spheres) and the N-terminal end (residue 8 in our model) used as an approximation for the RLuc attachment at the N-terminal end of β-arrestin 2 (green spheres), as the Rluc domain is absent in the simulated system. (B) Binding mode of the CXCR3 WT C-tail to β-arrestin 2. Negatively charged residues (Asp, Glu or phosphorylated Ser and Thr) on the C-tail are depicted in licorice and their Cα atoms are highlighted with red spheres. Positions mutated within this study are labeled. The inset provides a detailed depiction of the lariat loop region of β-arrestin 2 (blue) and interactions with negatively charged residues of the C-tail. Bar charts demonstrate the stability of polar interactions between K294 of the lariat loop and S358 and E359 of the C-tail during MD simulations. In the bar plots, systems with a low FlasH probe 4-5 resonance energy transfer (RET) are colored in red (WT and T360AS361A). System with an intermediate or high FlasH probe 4-5 RET are colored in black (4xA, S355A/S356A, L344X). (C) Distribution plots of the interdomain rotation angles of β-arrestin 2 in complex with C-tail mutants. The distribution of the interdomain rotation angles have been computed over the accumulated simulation frames for β-arrestin 2 in complex with C-tail of the WT versus mutants. The peaks of the plots indicate the most explored conformations for the WT system (WTpeak) versus mutant systems (e.g. L344Xpeak). Systems are grouped based on probe 4-5 RET. Intermediate and high probe 4-5 RET goes along with a shift of the distribution peak to lower rotation angles compared to the WT system (low probe 4-5 RET).