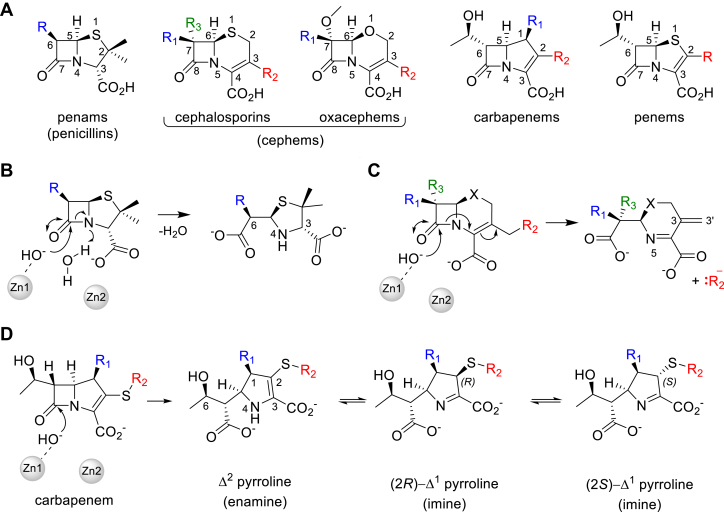
Figure 1.
Bicyclic β-lactams and their major hydrolysis products formed on reaction with metallo-β-lactamases.A, major classes of bicyclic β-lactam antibiotics, with variable substituent (R)-groups colored. B, penam/penicillin degradation by MBLs. In the resting state, a water/hydroxide bridges the dizinc MBL center. This becomes terminal to Zn1 and is activated to attack the β-lactam carbonyl, opening the β-lactam ring, with subsequent protonation of N-4. C, the degradation pathway of cephems with a C-3′ leaving group can result in loss of the C-3 R2 substituent, without protonation of the β-lactam N-5 nitrogen. D, tautomerization of carbapenem-derived Δ2 pyrroline hydrolysis products to give (R/S)-Δ1 pyrrolines via protonation at C-2.