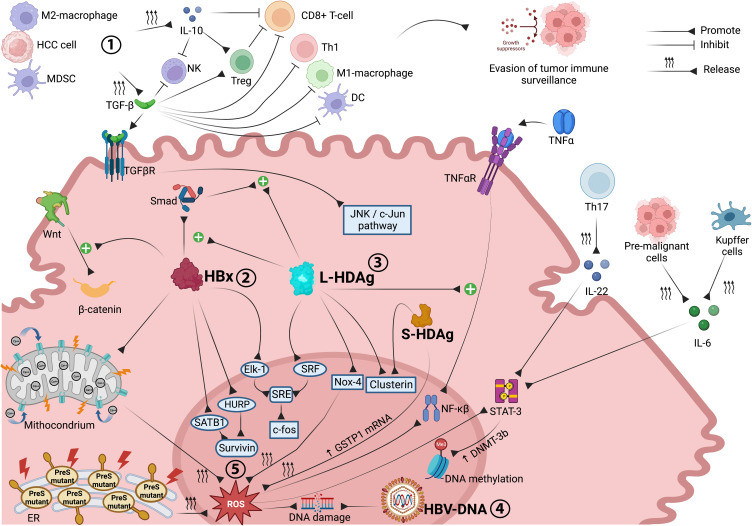
Figure 1.
Molecular interactions and oncogenic mechanisms occurring in the HCC microenvironment associated with chronic HBV-HDV infection. Both immune cell impairment and stimulation of pro-oncogenic intracellular signaling pathways are involved in CHB- and CDH-driven hepatocarcinogenesis. Through the release of TGF-β and IL-10, M2 macrophages, MDSCs and tumor cells inhibit the antitumor functions of immune effector cells, promoting tumor immune escape. HBx upregulates Wnt/β-catenin signaling and promotes survivin protein synthesis, supporting tumor cell proliferation and preventing apoptosis. Synergistically, L-HDAg and HBx trigger the JNK/c-Jun and SRE/c-fos cascades, both of which are involved in cancer cell growth, proliferation and survival. The integration of HBV-DNA into the host genome and the overproduction of ROS, triggered by HBx, L-HDAg and S-HDAg, promote DNA damage and genomic instability. ROS also promote cancer cell survival, angiogenesis and invasiveness through upregulation of the NF-ĸB pathway, which is also stimulated by L-HDAg in a TNFα-dependent manner, and STAT-3 signaling, which is further activated by Th17 (via IL-22), pre-malignant cells and Kupffer cells (via IL-6). Image created with Biorender.com.
Abbreviations: CDH, chronic delta hepatitis; CHB, chronic hepatitis B; DC, dendritic cells; DNMT-3b, DNA methyltransferase-3b; Elk-1, E26 transformation-specific like-1 protein; ER, endoplasmic reticulum; GSTP1, glutathione S-transferase P1; HBV, hepatitis B virus; HBx, X protein; HCC, hepatocellular carcinoma; HDV, hepatitis delta virus; HURP, hepatoma upregulated protein; IL-6, interleukin-6; IL-10, interleukin-10; IL-22, interleukin-22; JNK, c-Jun N-terminal kinase; L-HDAg, large hepatitis D antigen; MDSC, myeloid-derived suppressor cells; NK, natural killer cells; Nox-4, NADPH Oxidase 4; NF-ĸB, nuclear factor ĸB; ROS, reactive oxygen species; SATB1, special AT-rich sequence-binding protein-1; S-HDAg, small hepatitis D antigen; Smad, small mother against decapentaplegic proteins; SRE, serum response element; SRF, serum response factor; STAT3, signal transducer and activator of transcription 3; TGF-β, transforming growth factor-β; TGFβR, transforming growth factor-β receptor; Th1, T-helper 1 cells; Th17, T-helper 17 cells; TNFα, tumor necrosis factor α; TNFαR, tumor necrosis factor α receptor; Treg, regulatory T cells; Wnt, wingness-related integration site.