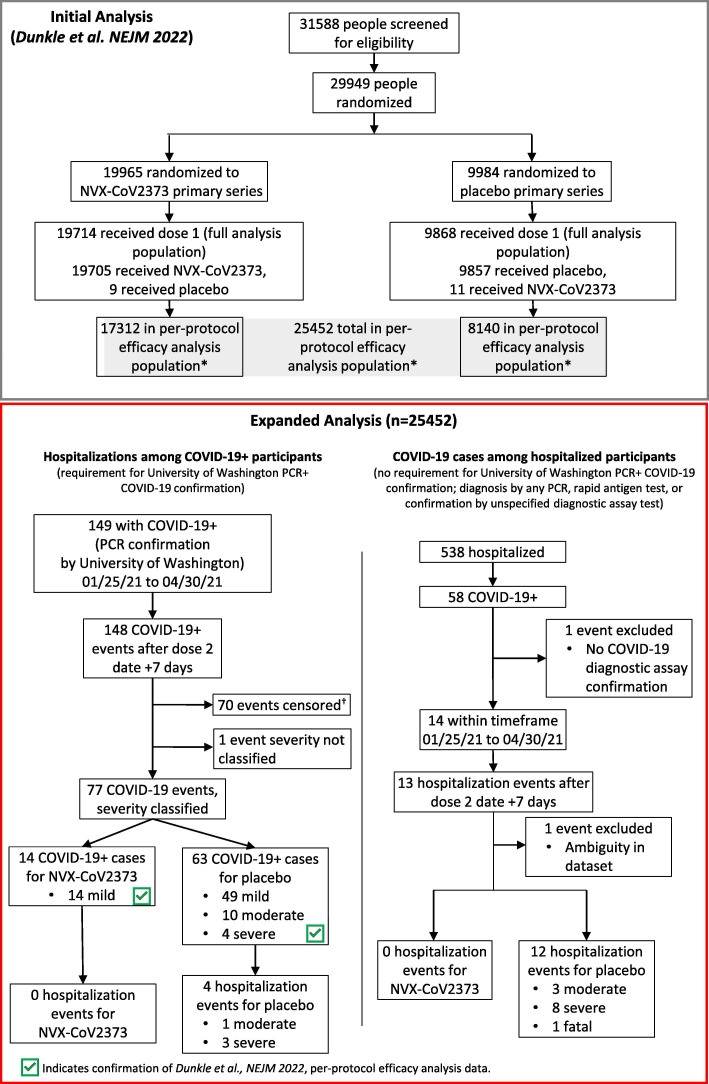
Fig. 1.
CONSORT Diagram for Original Trial and Expanded Analysis. CONSORT diagram for original trial participants and event identification in the expanded analysis. The 4 identified hospitalized participants are among the 77 events analyzed for the PREVENT-19 per-protocol primary efficacy analysis endpoint. COVID-19+ indicated diagnosis of COVID-19. *Participants received both doses as assigned, were seronegative for anti–severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) nucleocapsid protein and had a SARS-CoV-2 RNA reverse transcriptase–polymerase chain reaction (RT-PCR)–negative nasal swab at baseline and did not have a censoring event at any time before 7 days after the second injection. †Censoring events for exclusion of participants from the per-protocol analysis population as described by Dunkle et al. [20] NEJM 2022 (Supplemental S1.3).