Fig. 5.
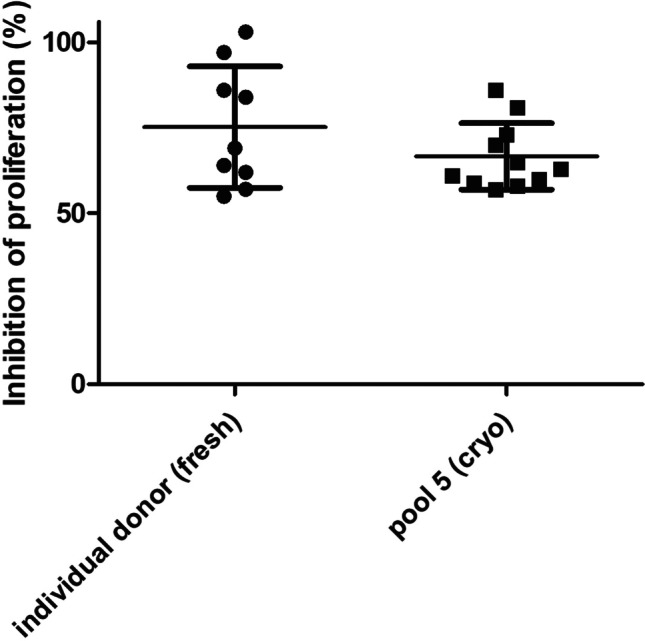
Comparability of pooled vs. individual PBMC in the assessment of immunopotency of clinical batches of MSC,WJ drug products derived from the same Master Cell Bank. Comparability study conducted on immunoptency assays of clinical-grade batches MSC,WJ used in the context of ongoing Phase I/IIa trials (EudraCT No.2021–000,346-18, Clinicaltrials.gov Id. NCT05054803; EudraCT No. 2020–001,505-22, ClinicalTrials.gov Id. NCT04390139; and EudraCT No. 2018–001,964-49, ClinicalTrials.gov Id. NCT03798353) and compassionate uses (in the management of graft versus host disease, GvHD). No statistically significant differences were observed between the potency assays performed on the group using individual donor of fresh PBMC (n = 9) and the group using cryopreserved pooled PBMC from 5 different donors (n = 11) (P = 0.1858; unpaired t test). Interestingly the range of inhibition values was smaller showing lower variability in the group using pooled PBMC
