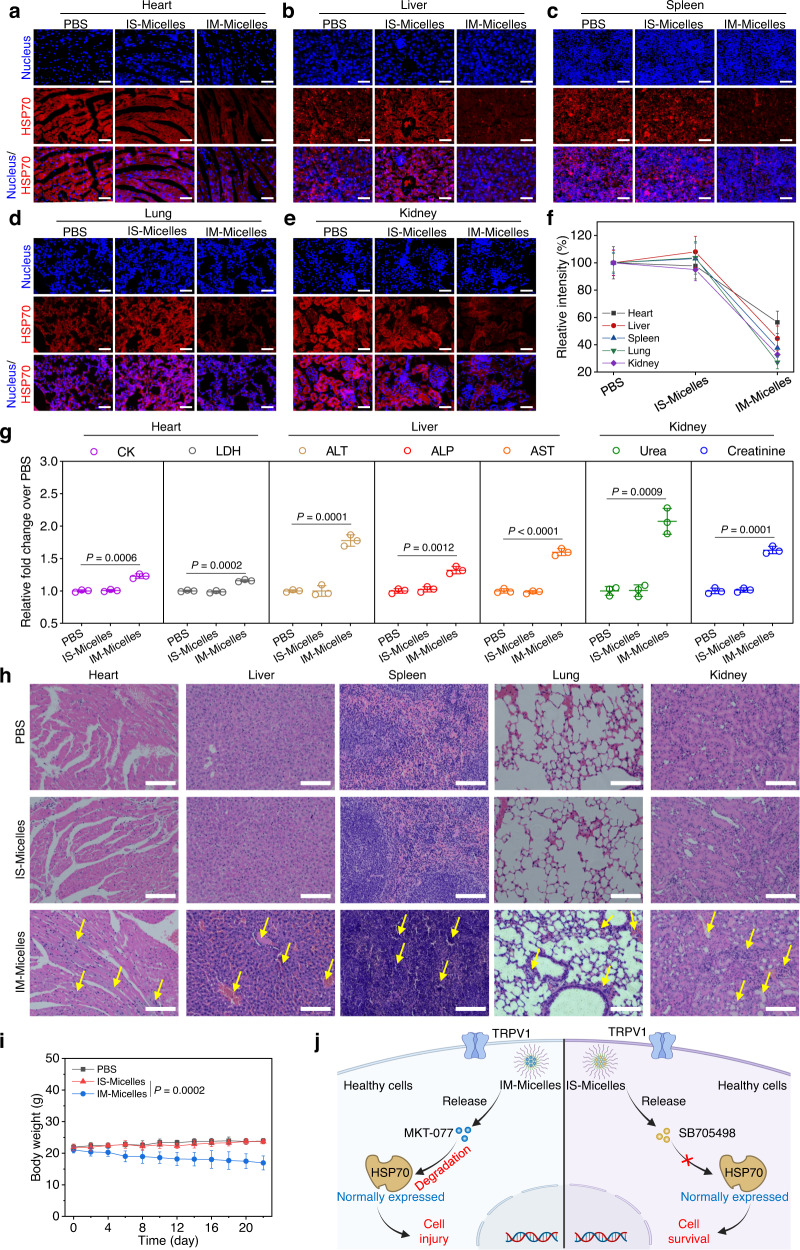
Fig. 5. TRPV1 blockade confers superior safety without interfering normally expressed HSP70 in healthy tissues.
CLSM images of HSP70 (Alexa Fluor 594-labeled anti-HSP70 antibody, red) in different sections of non-cancerous tissues, including heart (a), liver (b), spleen (c), lung (d), kidney (e), and corresponding fluorescence intensity analysis (f) at 24 h post-injection of IS-Micelles or IM-Micelles at the dose of 5.0 mg kg−1 SB705498 or 5.0 mg kg−1 MKT-077 (equal to 7.5 mg kg−1 ICG) using immunofluorescence staining (n = 3 independent experiments). Scale bars, 100 μm. g Blood levels of heart function markers (CK and LDH), hepatic function markers (ALP, ALT and AST) and renal function markers (Urea and Creatinine) treated with 5.0 mg kg−1 SB705498 (IS-Micelles) or MKT-077 (IM-Micelles) at 3 days post-injection (n = 3 biological replicates). h H&E images of normal tissues from the mice at 3 days after intravenous injection of 5.0 mg kg−1 SB705498 (IS-Micelles) or MKT-077 (IM-Micelles). Yellow arrows represent the histological damage such as fibrosis in different tissues, and renal tubular dilution in kidneys. Scale bars, 200 μm. i Body weights of the mice after intravenous injection of 5.0 mg kg−1 SB705498 (IS-Micelles) or MKT-077 (IM-Micelles) during 22 days (n = 5 mice per group). j Schematic illustration of TRPV1 blockade-mediated superior safety without interference on normally expressed HSP70 of healthy cells using IS-Micelles (right), as compared to IM-Micelles (left) which led to inevitable degradation of normally expressed HSP70 with severe concurrent toxicities. Data are presented as mean ± SD (f, g, i). Statistical significance was determined by one-way ANOVA with Tukey’s post hoc test. The experiments for a–e, and h were repeated three times independently with similar results. Source data are provided as a Source Data file.